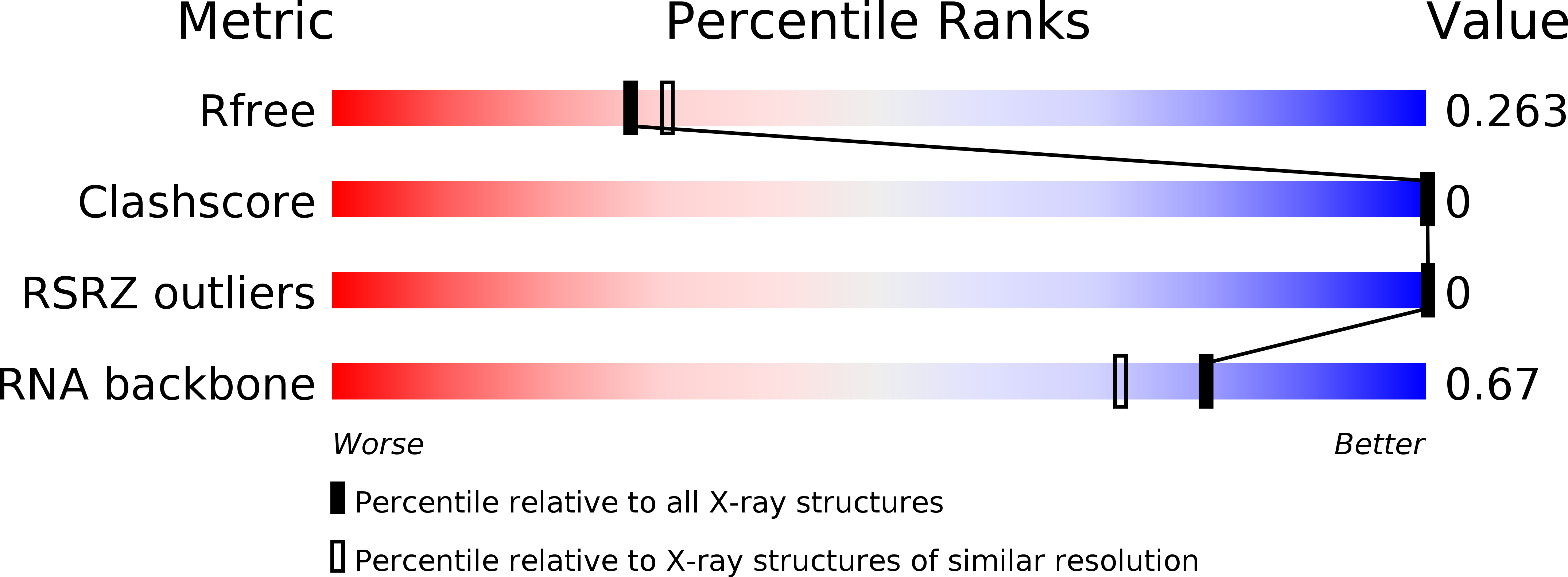Deposition Date
2013-08-28
Release Date
2013-11-06
Last Version Date
2023-12-20
Entry Detail
PDB ID:
4C40
Keywords:
Title:
The molecular recognition of kink turn structure by the L7Ae class of proteins
Biological Source:
Source Organism(s):
HALOARCULA MARISMORTUI (Taxon ID: 2238)
Method Details:
Experimental Method:
Resolution:
2.20 Å
R-Value Free:
0.26
R-Value Work:
0.22
Space Group:
P 63 2 2