Deposition Date
2013-08-07
Release Date
2013-08-21
Last Version Date
2023-12-20
Entry Detail
PDB ID:
4C0R
Keywords:
Title:
Molecular and structural basis of glutathione import in Gram-positive bacteria via GshT and the cystine ABC importer TcyBC of Streptococcus mutans.
Biological Source:
Source Organism(s):
STREPTOCOCCUS MUTANS (Taxon ID: 1309)
Expression System(s):
Method Details:
Experimental Method:
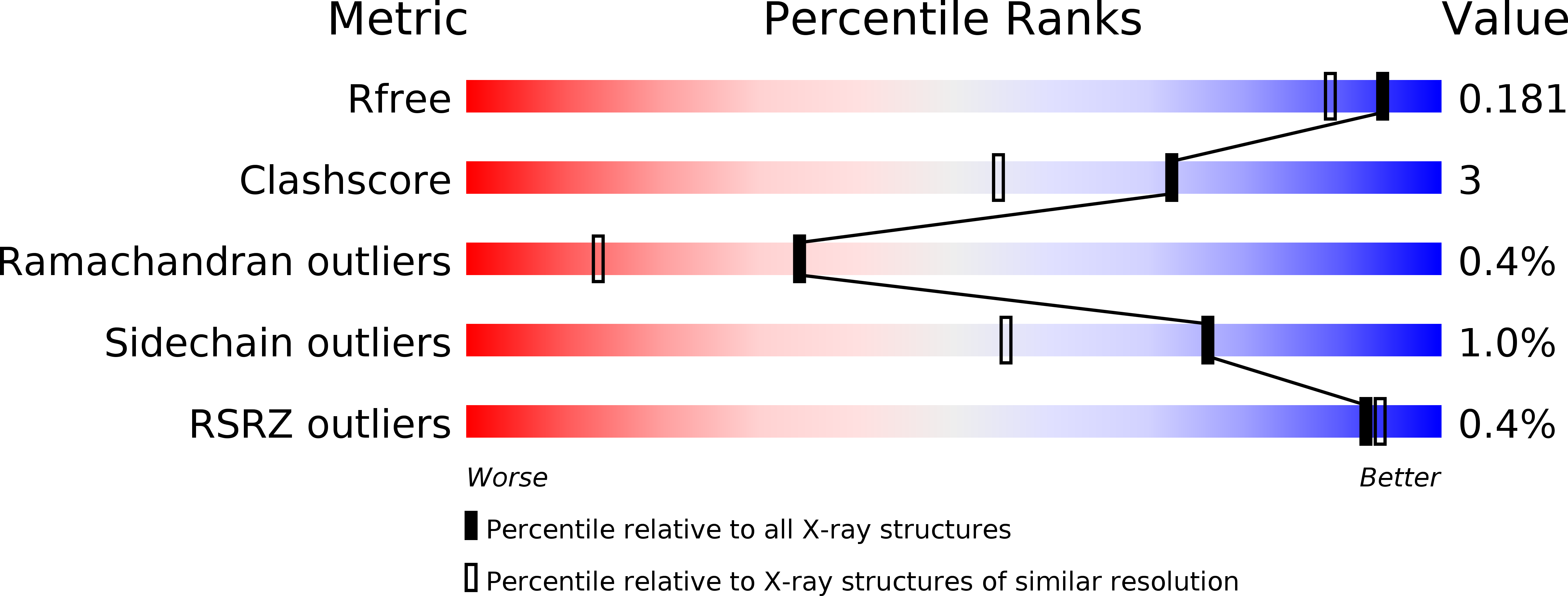
Resolution:
1.55 Å
R-Value Free:
0.18
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 1 21 1