Deposition Date
2013-07-22
Release Date
2014-04-23
Last Version Date
2024-05-08
Entry Detail
PDB ID:
4BYZ
Keywords:
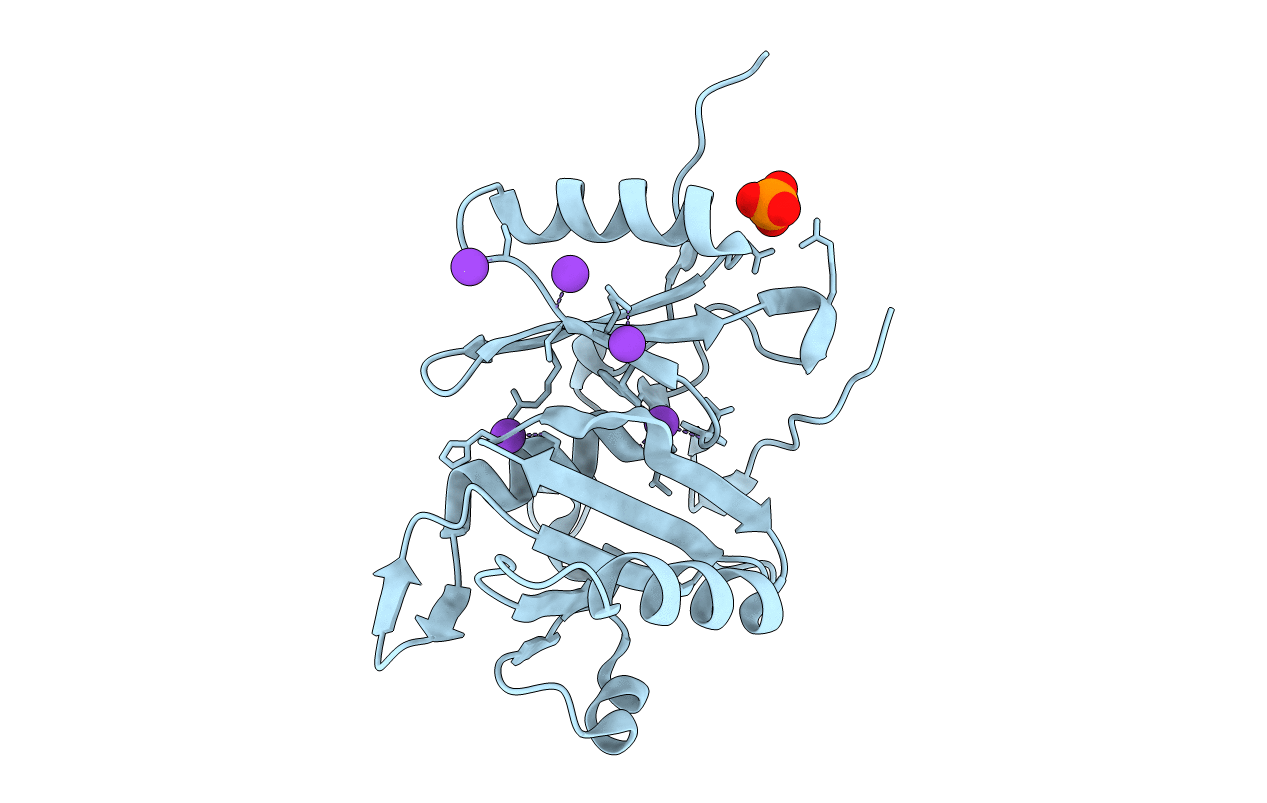
Title:
Structural characterization using Sulfur-SAD of the cytoplasmic domain of Burkholderia pseudomallei PilO2Bp, an actin-like protein component of a Type IVb R64-derivative pilus machinery.
Biological Source:
Source Organism(s):
BURKHOLDERIA PSEUDOMALLEI (Taxon ID: 272560)
Expression System(s):
Method Details:
Experimental Method:
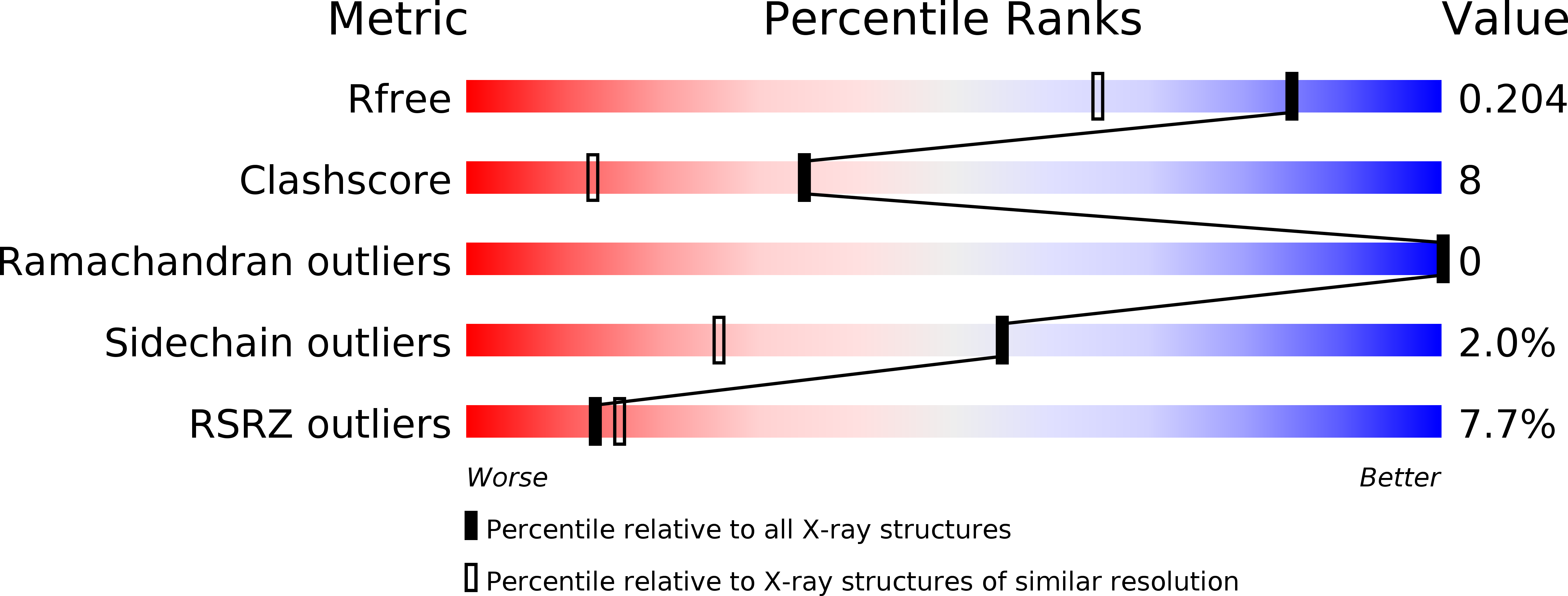
Resolution:
1.55 Å
R-Value Free:
0.19
R-Value Work:
0.14
R-Value Observed:
0.15
Space Group:
P 41 21 2