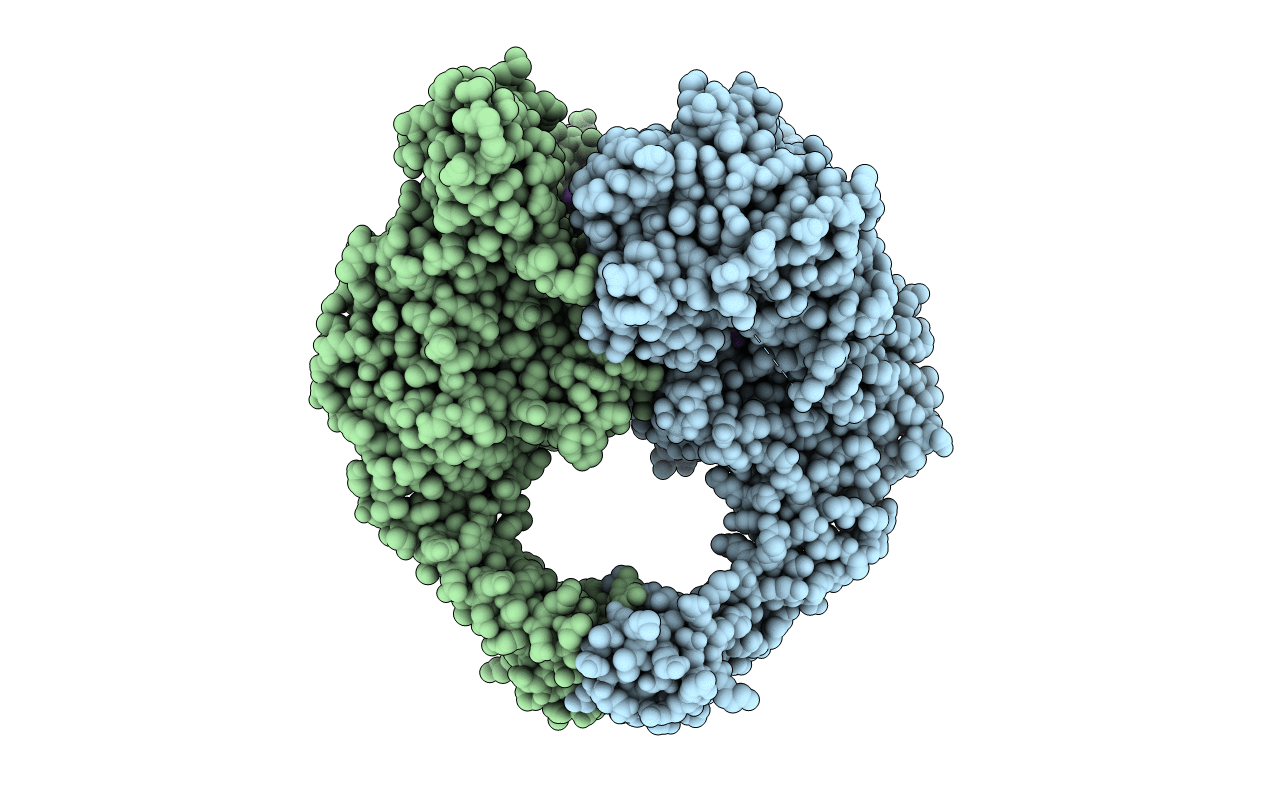
Deposition Date
2013-06-20
Release Date
2013-08-07
Last Version Date
2023-12-20
Entry Detail
PDB ID:
4BUL
Keywords:
Title:
Novel hydroxyl tricyclics (e.g. GSK966587) as potent inhibitors of bacterial type IIA topoisomerases
Biological Source:
Source Organism(s):
STAPHYLOCOCCUS AUREUS (Taxon ID: 1280)
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
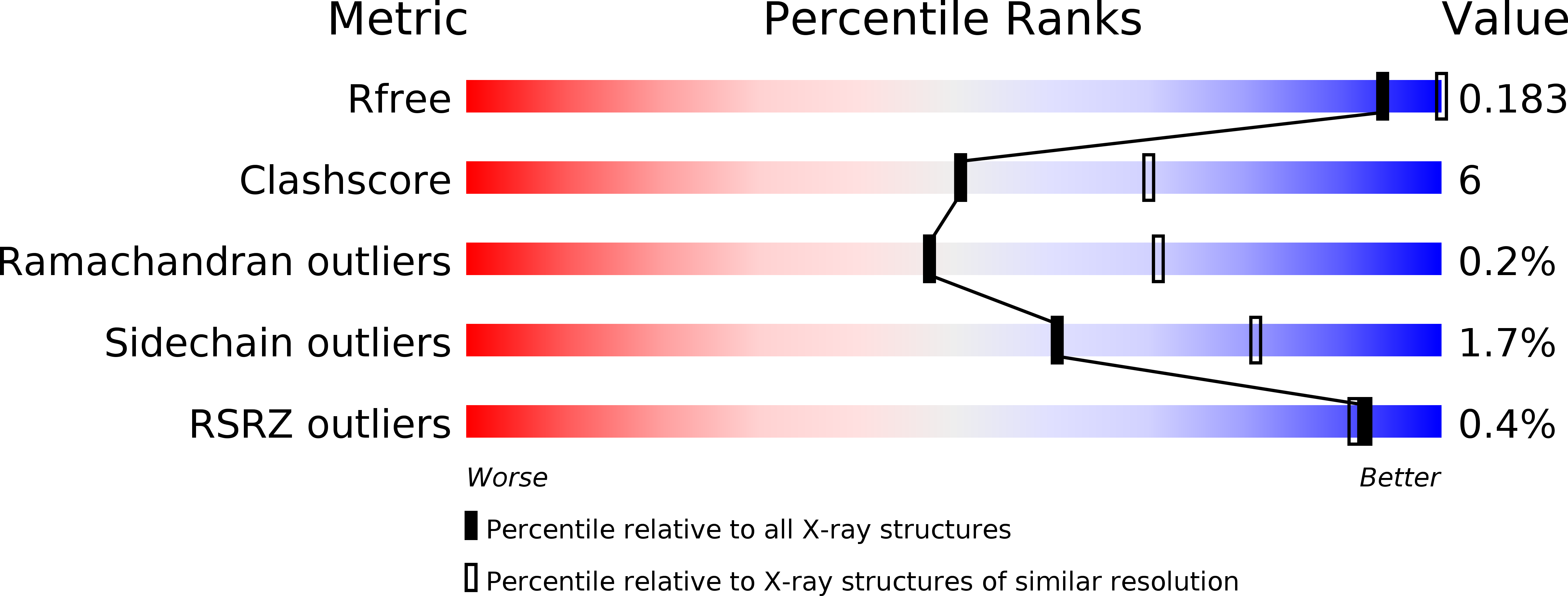
Resolution:
2.60 Å
R-Value Free:
0.18
R-Value Work:
0.14
R-Value Observed:
0.14
Space Group:
P 61