Deposition Date
2013-06-18
Release Date
2014-01-22
Last Version Date
2024-10-16
Entry Detail
PDB ID:
4BTH
Keywords:
Title:
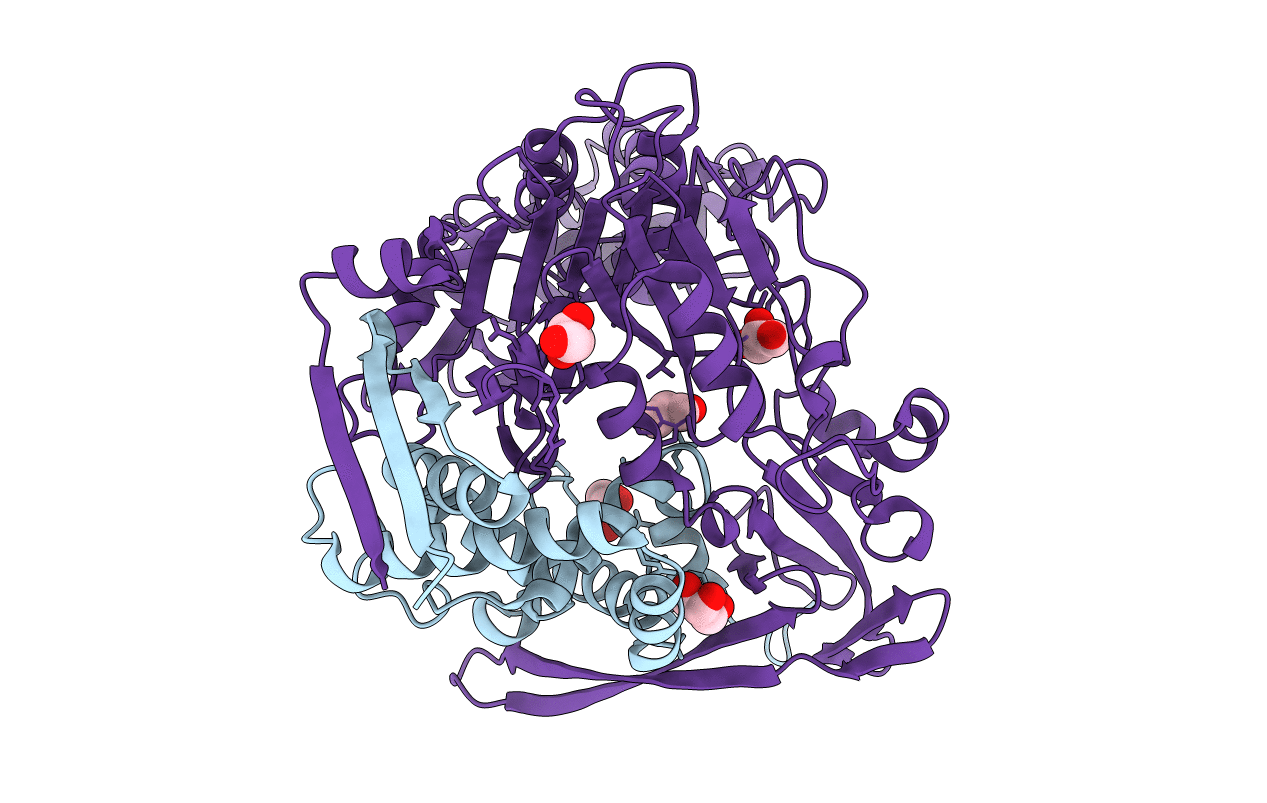
The LeuA146Trp,PheB24Tyr Double Mutant of the Quorum Quenching N-acyl Homoserine Lactone Acylase PvdQ Has an Altered Substrate Specificity Towards Small Acyl Chains
Biological Source:
Source Organism(s):
PSEUDOMONAS AERUGINOSA (Taxon ID: 208964)
Expression System(s):
Method Details:
Experimental Method:
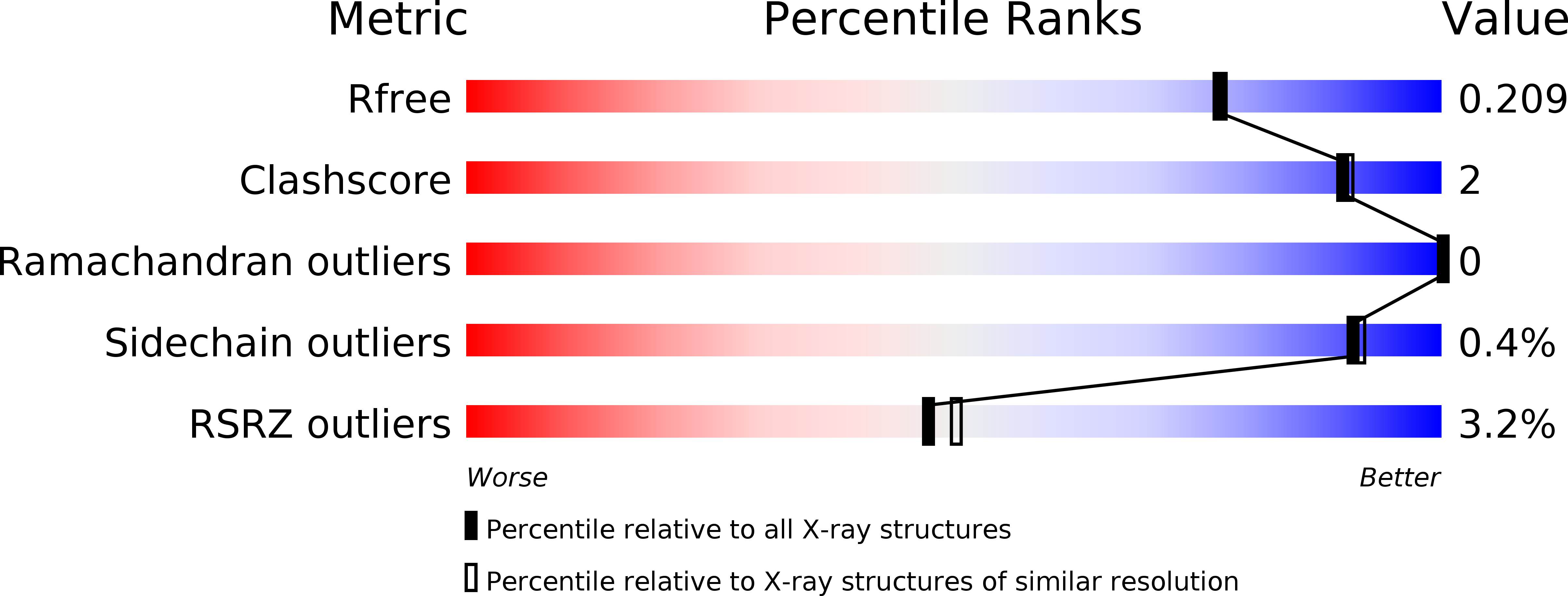
Resolution:
1.90 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.18
Space Group:
C 2 2 21