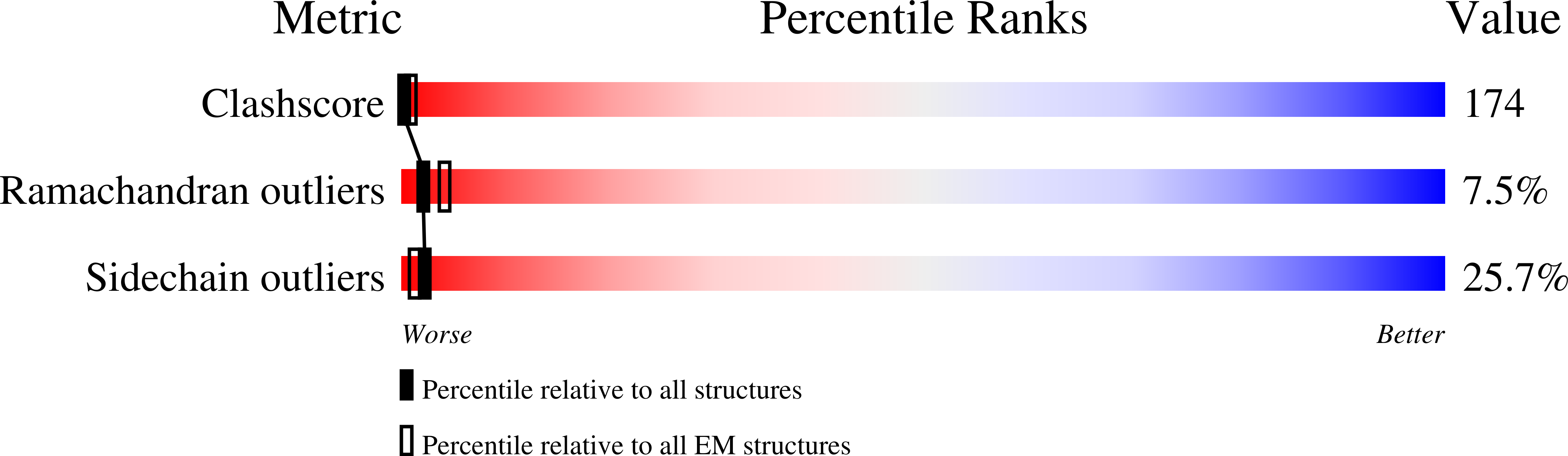
Deposition Date
2013-05-22
Release Date
2013-06-26
Last Version Date
2024-10-23
Entry Detail
PDB ID:
4BOR
Keywords:
Title:
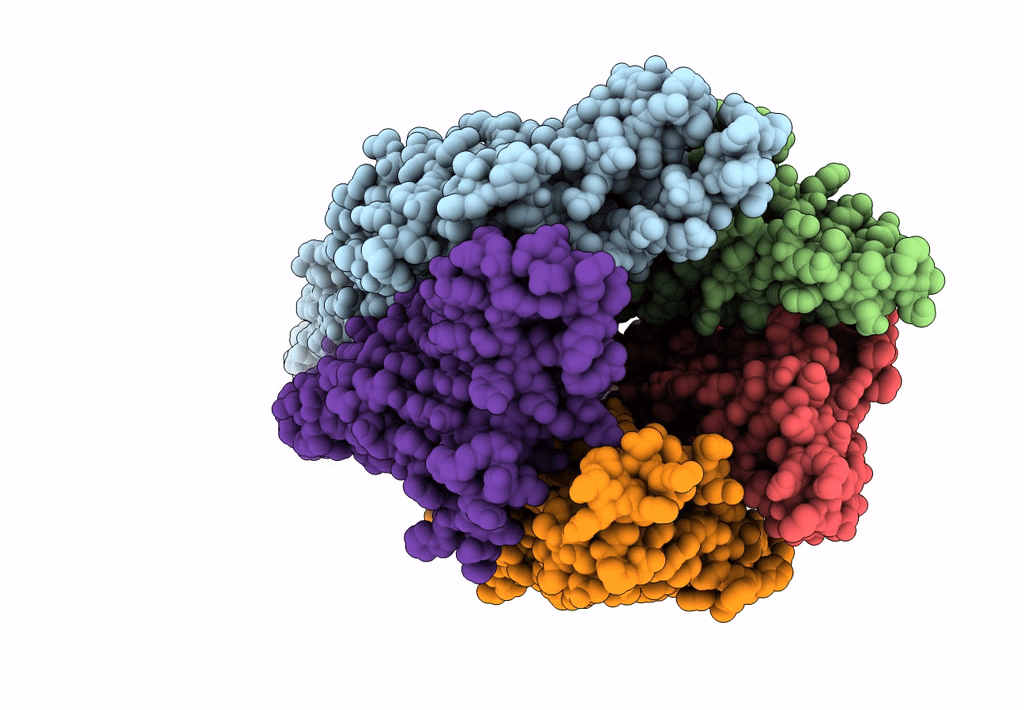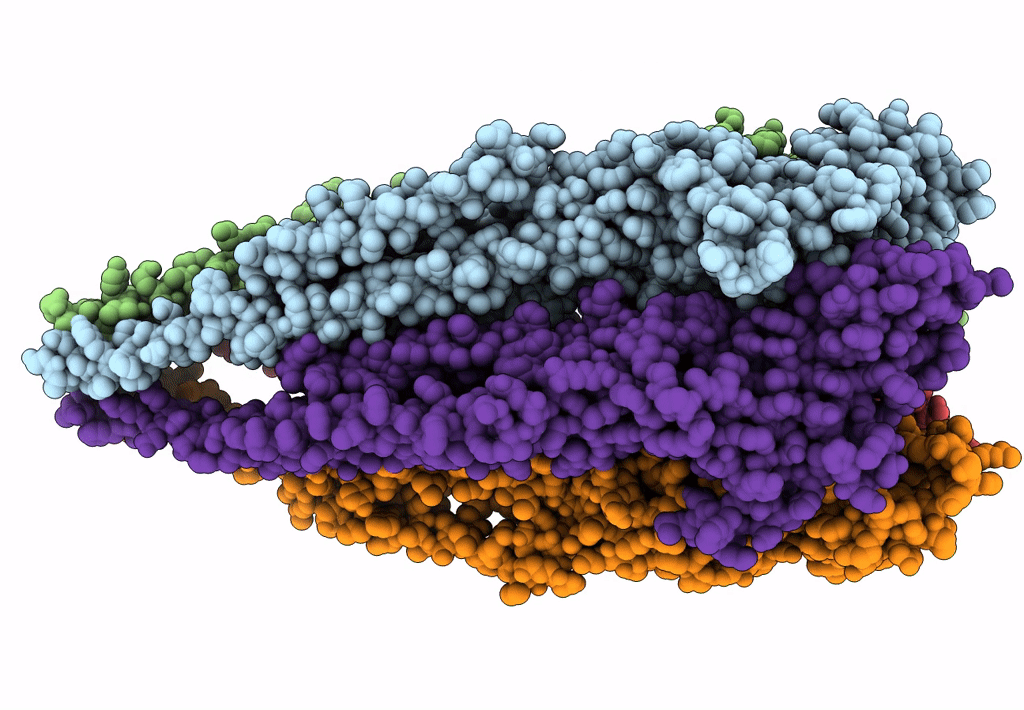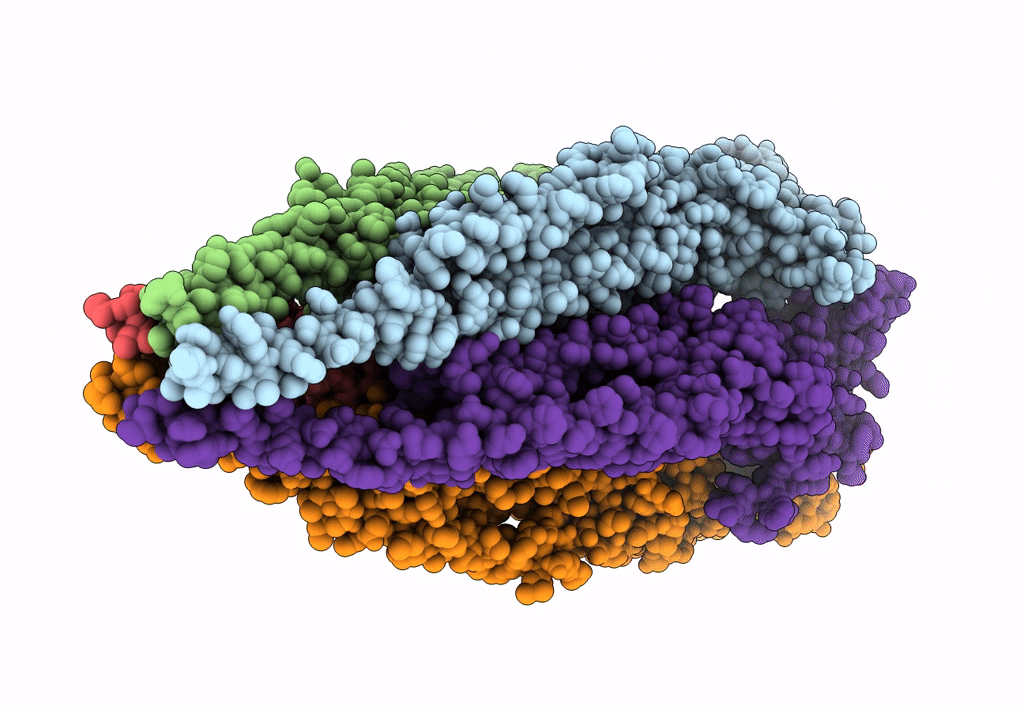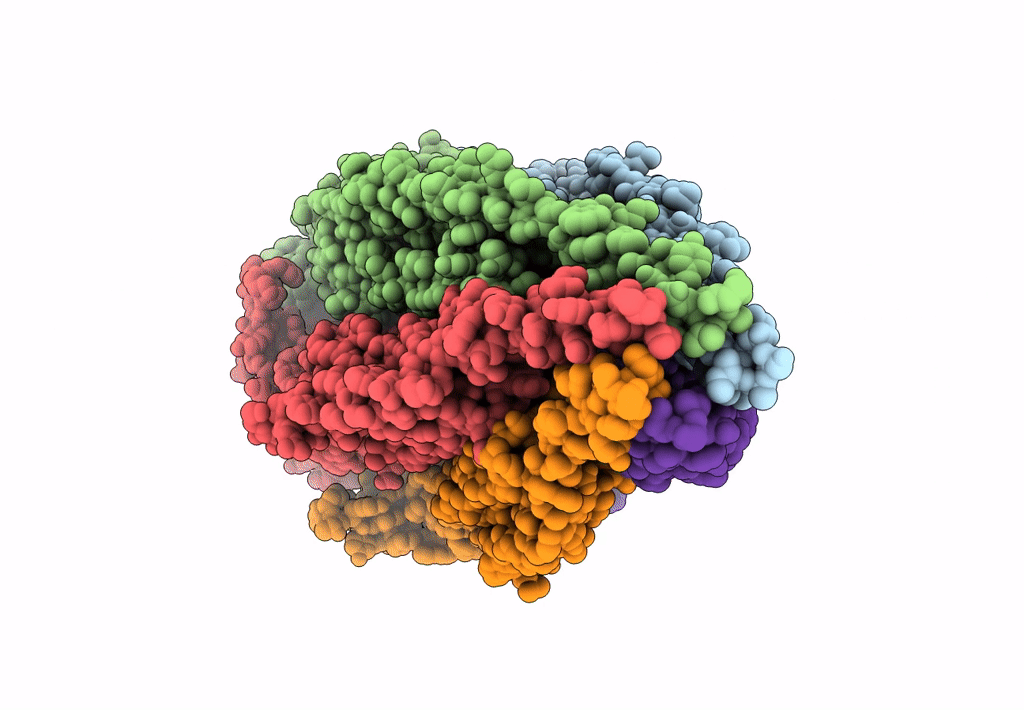
The structure and super-organization of acetylcholine receptor-rapsyn complexes class D
Biological Source:
Source Organism(s):
TORPEDO MARMORATA (Taxon ID: 7788)
Method Details:
Experimental Method:
Resolution:
42.00 Å
Aggregation State:
TISSUE
Reconstruction Method:
TOMOGRAPHY