Deposition Date
2012-09-04
Release Date
2013-09-11
Last Version Date
2023-12-20
Entry Detail
PDB ID:
4B9C
Keywords:
Title:
Biomass sensoring modules from putative Rsgi-like proteins of Clostridium thermocellum resemble family 3 carbohydrate-binding module of cellulosome
Biological Source:
Source Organism(s):
CLOSTRIDIUM THERMOCELLUM (Taxon ID: 203119)
Expression System(s):
Method Details:
Experimental Method:
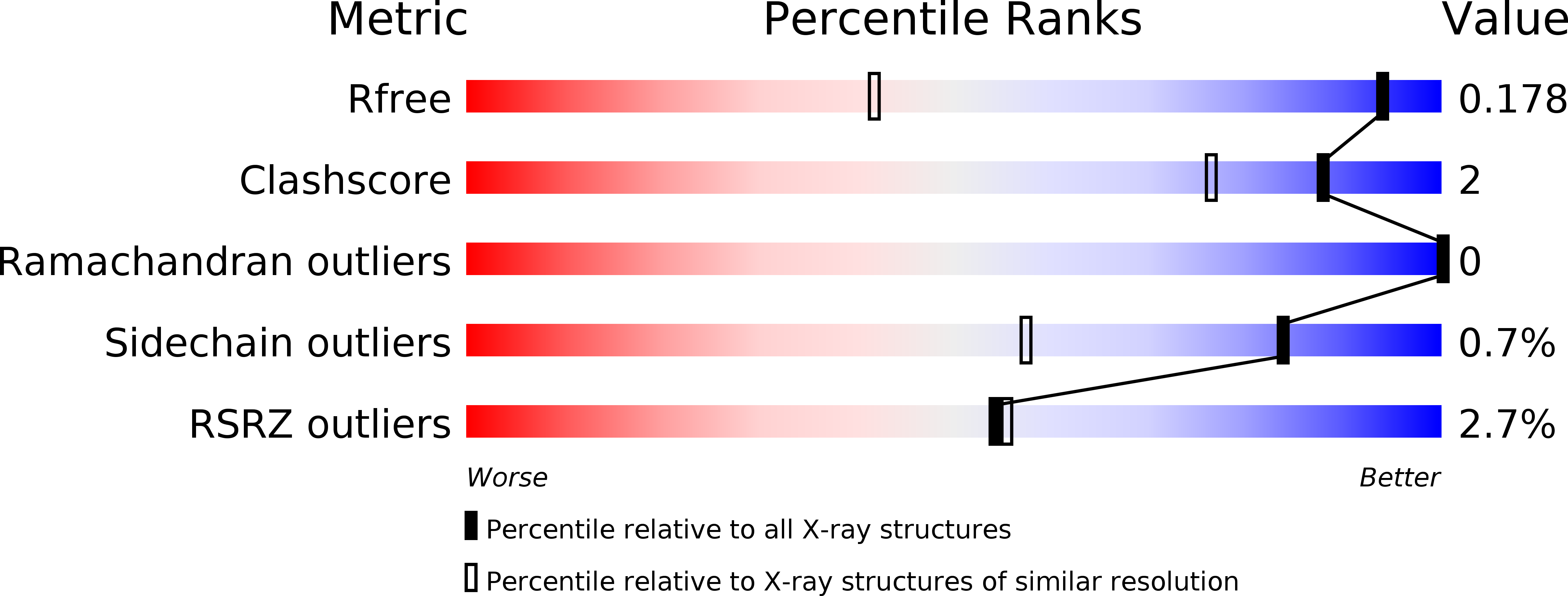
Resolution:
1.17 Å
R-Value Free:
0.18
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
P 41