Deposition Date
2012-06-15
Release Date
2012-08-15
Last Version Date
2024-11-13
Entry Detail
PDB ID:
4AXY
Keywords:
Title:
A molecular basis for the action of the collagen-specific chaperone Hsp47-SERPINH1 and its structure-specific client recognition.
Biological Source:
Source Organism(s):
SYNTHETIC CONSTRUCT (Taxon ID: 32630)
Method Details:
Experimental Method:
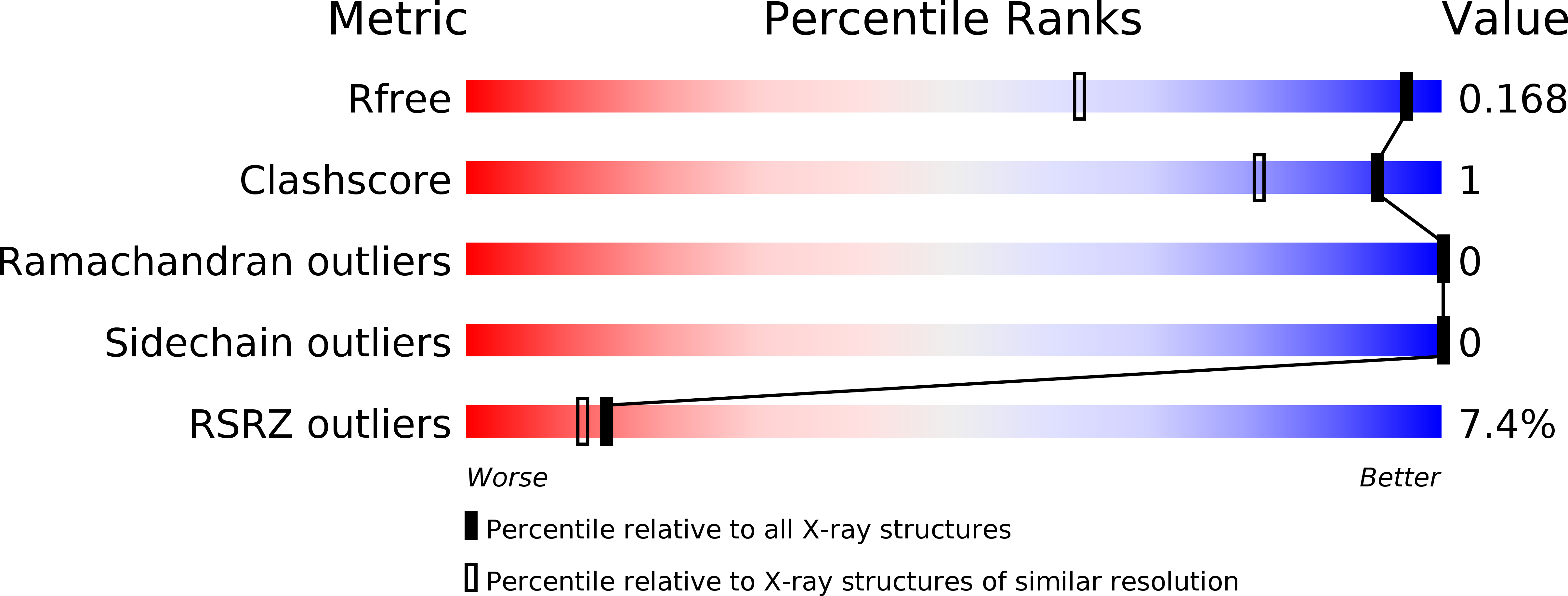
Resolution:
1.24 Å
R-Value Free:
0.17
R-Value Work:
0.14
R-Value Observed:
0.15
Space Group:
P 43 21 2