Deposition Date
2012-05-21
Release Date
2012-08-15
Last Version Date
2023-12-20
Entry Detail
PDB ID:
4AUQ
Keywords:
Title:
Structure of BIRC7-UbcH5b-Ub complex.
Biological Source:
Source Organism:
HOMO SAPIENS (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
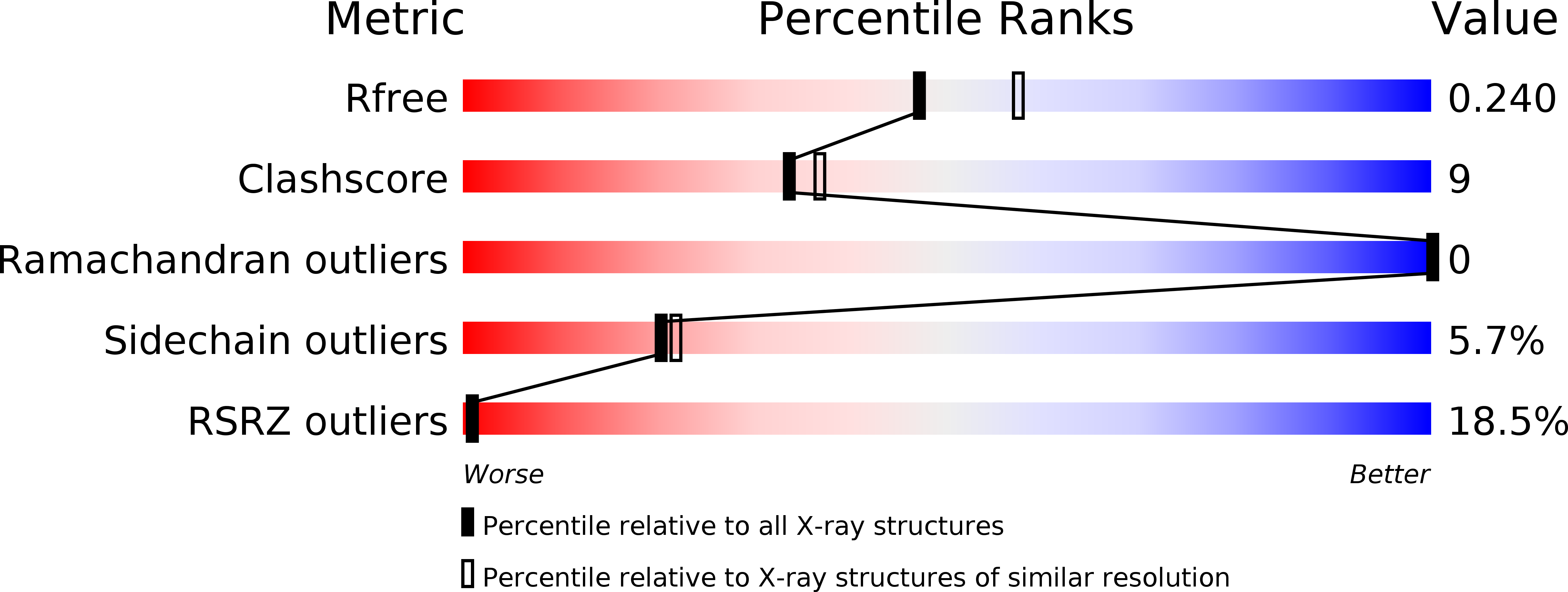
Resolution:
2.18 Å
R-Value Free:
0.24
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 43 21 2