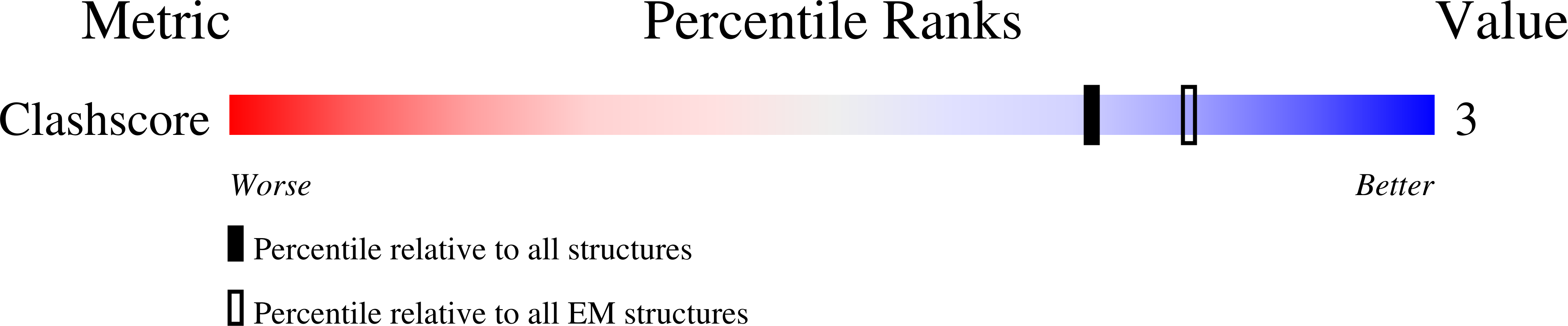Deposition Date
2012-04-19
Release Date
2012-11-21
Last Version Date
2024-05-08
Entry Detail
PDB ID:
4AQV
Keywords:
Title:
Model of human kinesin-5 motor domain (3HQD) and mammalian tubulin heterodimer (1JFF) docked into the 9.7-angstrom cryo-EM map of microtubule-bound kinesin-5 motor domain in the AMPPPNP state.
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
BOS TAURUS (Taxon ID: 9913)
BOS TAURUS (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
9.70 Å
Aggregation State:
FILAMENT
Reconstruction Method:
SINGLE PARTICLE