Deposition Date
2012-01-30
Release Date
2012-12-26
Last Version Date
2023-12-20
Entry Detail
PDB ID:
4AGK
Keywords:
Title:
Crystal structure of capsid protein (110-267) from Aura virus
Biological Source:
Source Organism(s):
AURA VIRUS (Taxon ID: 44158)
Expression System(s):
Method Details:
Experimental Method:
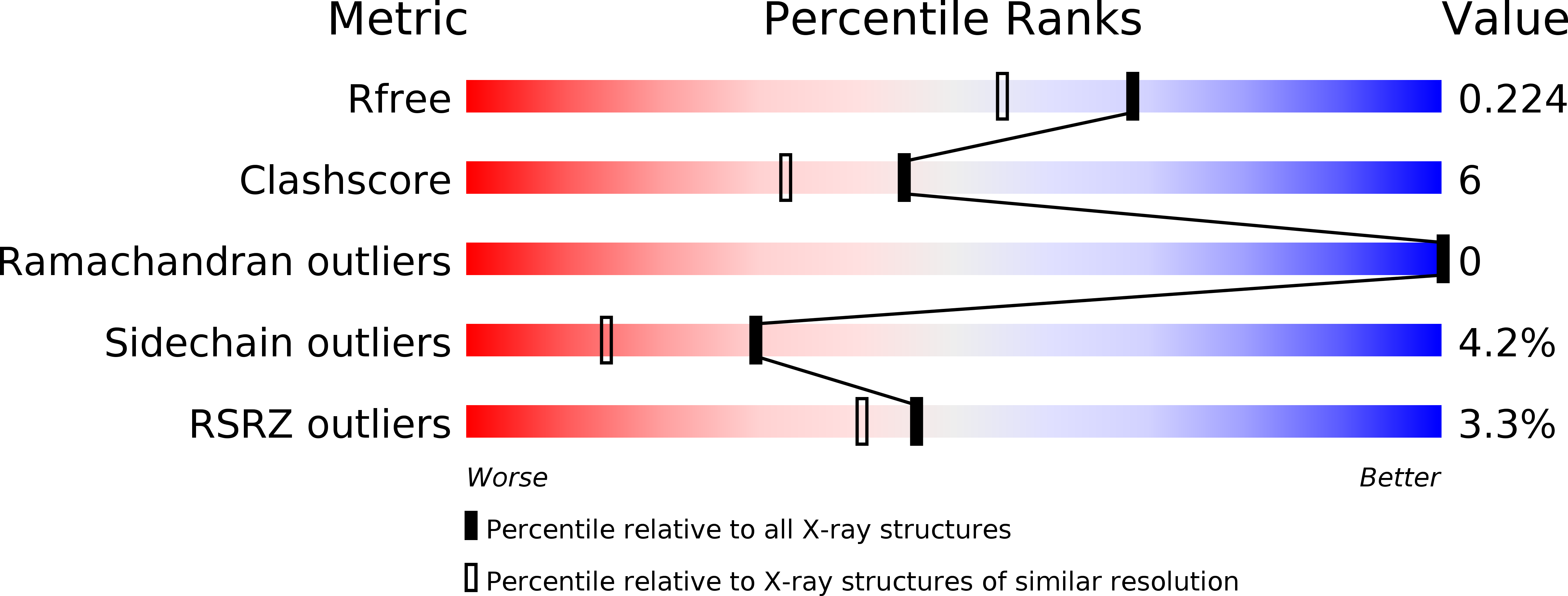
Resolution:
1.81 Å
R-Value Free:
0.23
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1