Deposition Date
2011-10-28
Release Date
2012-11-14
Last Version Date
2024-05-08
Entry Detail
PDB ID:
4A5U
Keywords:
Title:
Turnip yellow mosaic virus proteinase and Escherichia coli 30S ribosomal S15
Biological Source:
Source Organism(s):
TURNIP YELLOW MOSAIC VIRUS (Taxon ID: 12154)
ESCHERICHIA COLI (Taxon ID: 469008)
ESCHERICHIA COLI (Taxon ID: 469008)
Expression System(s):
Method Details:
Experimental Method:
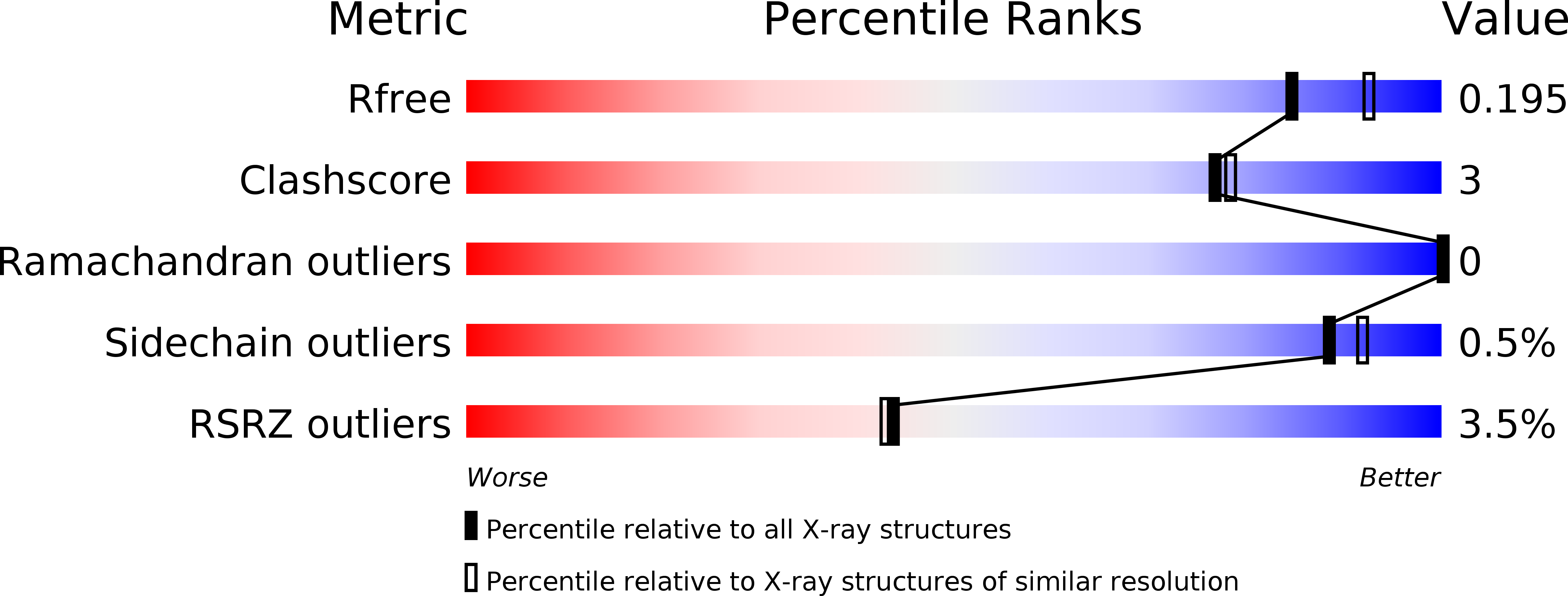
Resolution:
2.00 Å
R-Value Free:
0.20
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
P 31 2 1