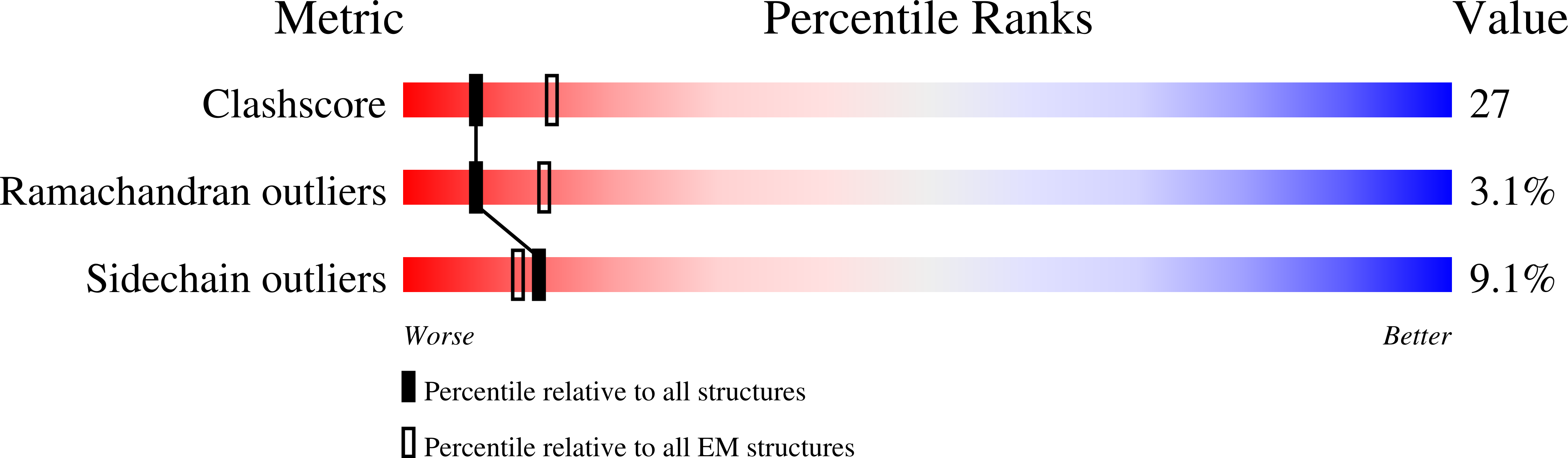
Deposition Date
2000-02-23
Release Date
2000-04-10
Last Version Date
2024-11-13
Entry Detail
PDB ID:
487D
Keywords:
Title:
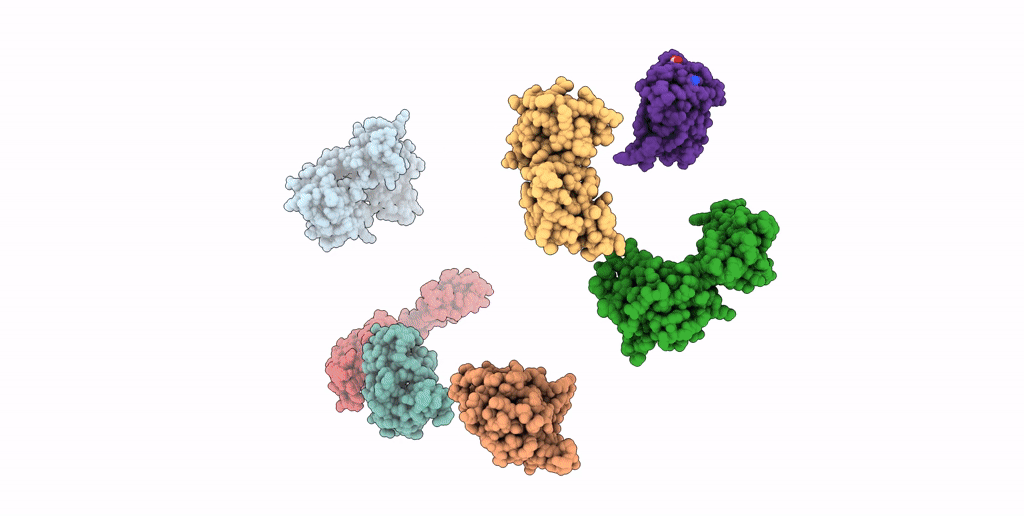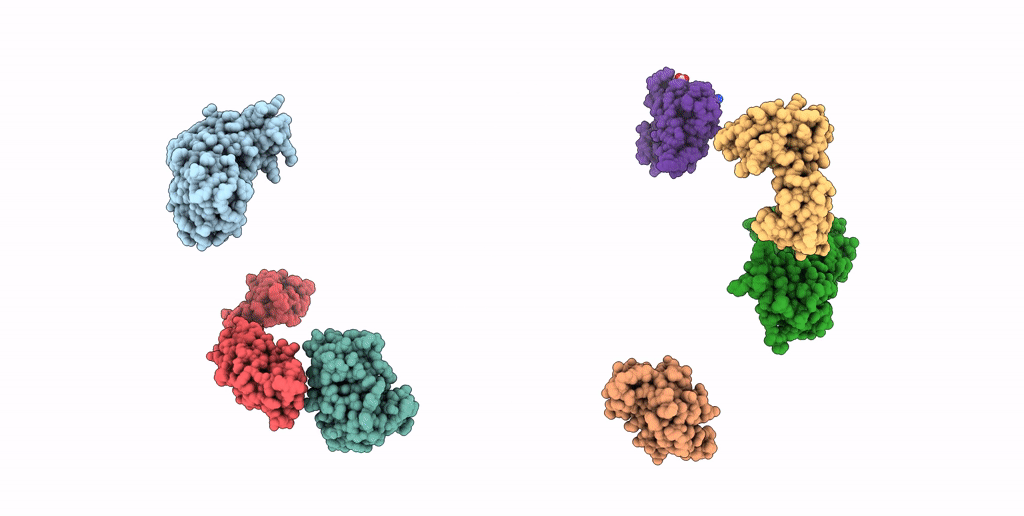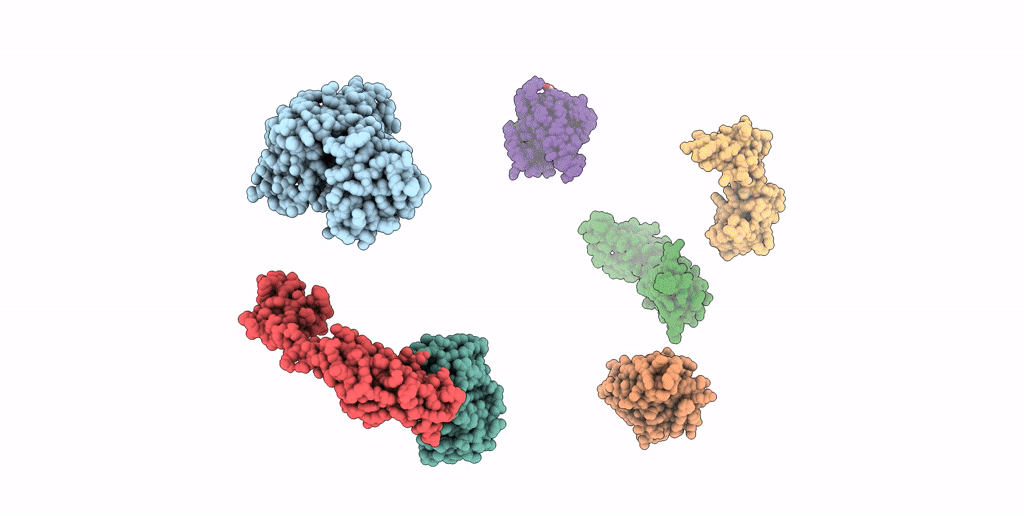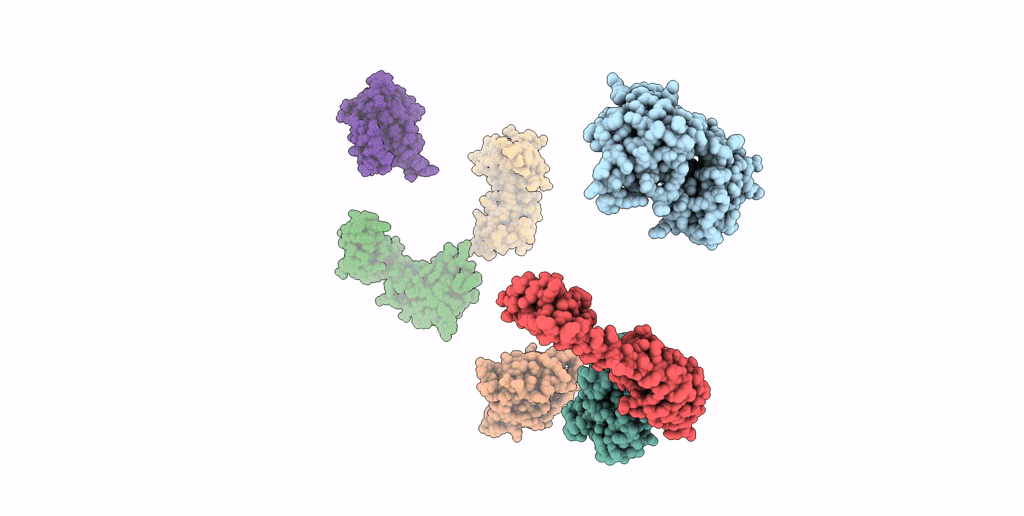
SEVEN RIBOSOMAL PROTEINS FITTED TO A CRYO-ELECTRON MICROSCOPIC MAP OF THE LARGE 50S SUBUNIT AT 7.5 ANGSTROMS RESOLUTION
Biological Source:
Source Organism(s):
Thermus thermophilus (Taxon ID: 274)
Geobacillus stearothermophilus (Taxon ID: 1422)
Thermotoga maritima (Taxon ID: 2336)
Escherichia coli (Taxon ID: 562)
Geobacillus stearothermophilus (Taxon ID: 1422)
Thermotoga maritima (Taxon ID: 2336)
Escherichia coli (Taxon ID: 562)
Method Details:
Experimental Method:
Resolution:
7.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE