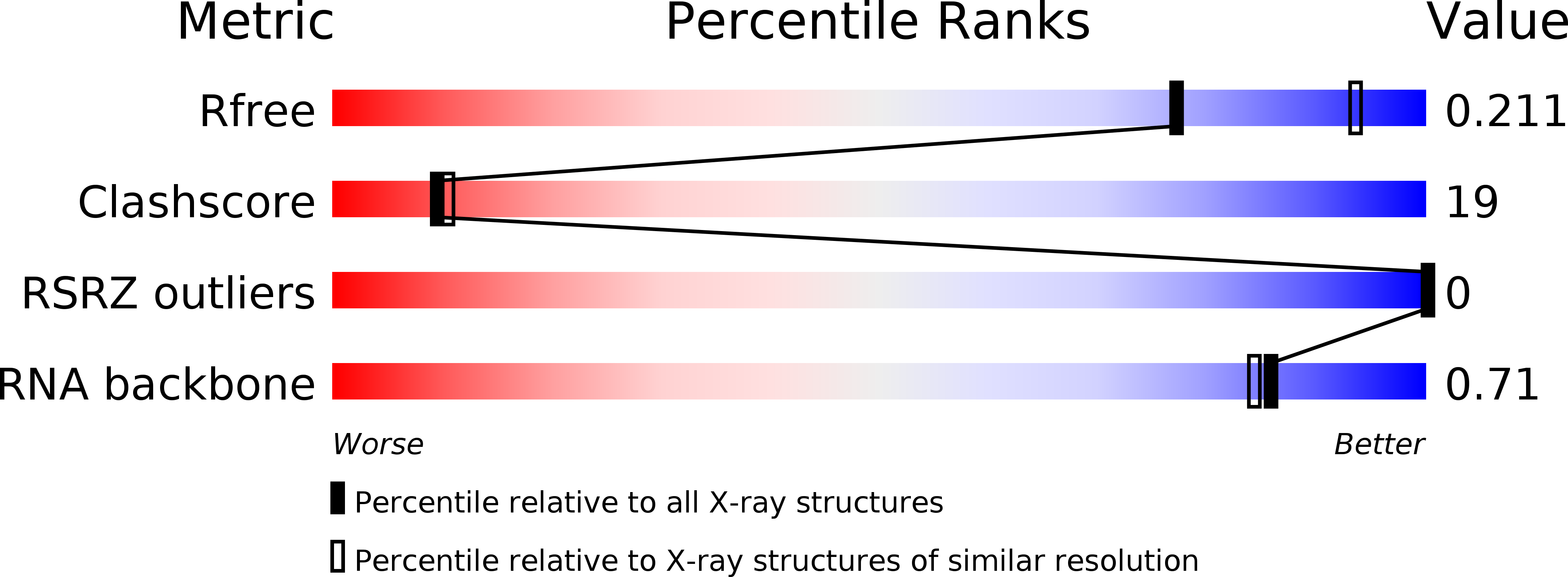Deposition Date
1999-03-18
Release Date
1999-12-02
Last Version Date
2023-12-27
Entry Detail
PDB ID:
462D
Keywords:
Title:
CRYSTAL STRUCTURE OF THE HIV-1 GENOMIC RNA DIMERIZATION INITIATION SITE
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Free:
0.21
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 31 2 1