Deposition Date
2011-06-13
Release Date
2012-10-03
Last Version Date
2023-12-20
Entry Detail
PDB ID:
3ZR4
Keywords:
Title:
STRUCTURAL EVIDENCE FOR AMMONIA TUNNELING ACROSS THE (BETA-ALPHA)8 BARREL OF THE IMIDAZOLE GLYCEROL PHOSPHATE SYNTHASE BIENZYME COMPLEX
Biological Source:
Source Organism(s):
THERMOTOGA MARITIMA (Taxon ID: 2336)
Expression System(s):
Method Details:
Experimental Method:
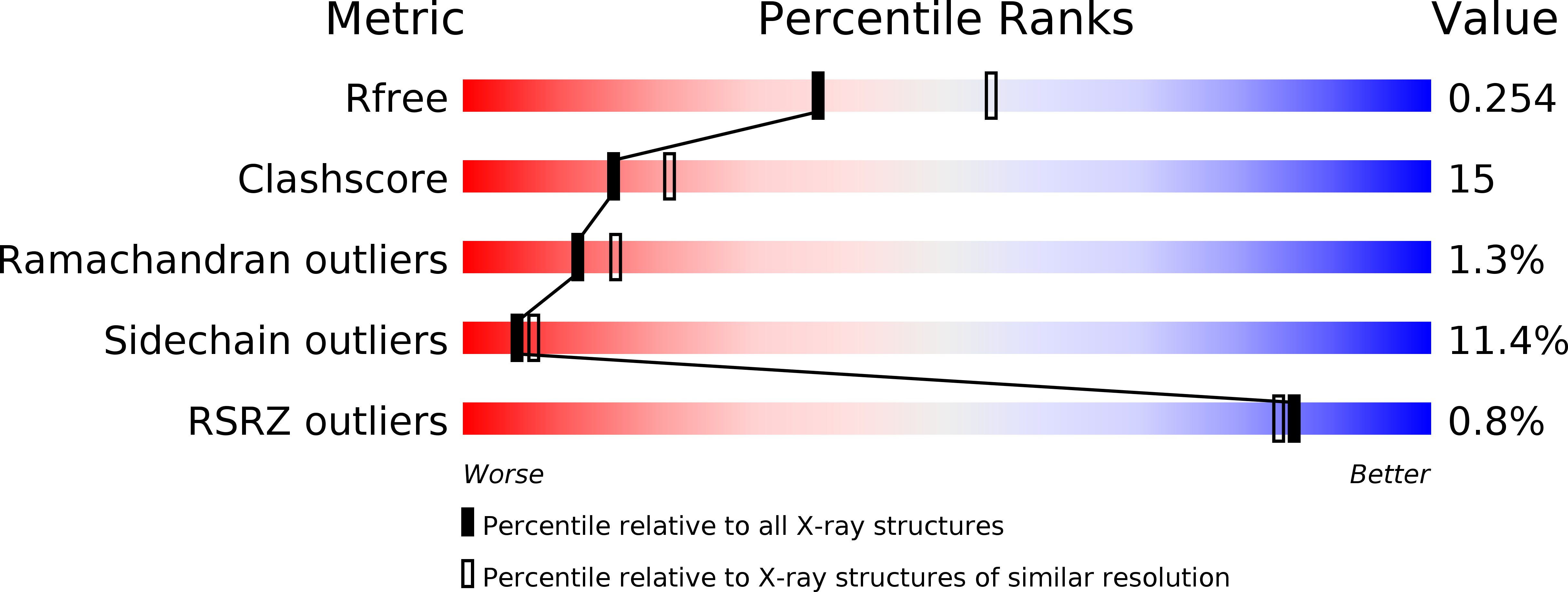
Resolution:
2.41 Å
R-Value Free:
0.25
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 32