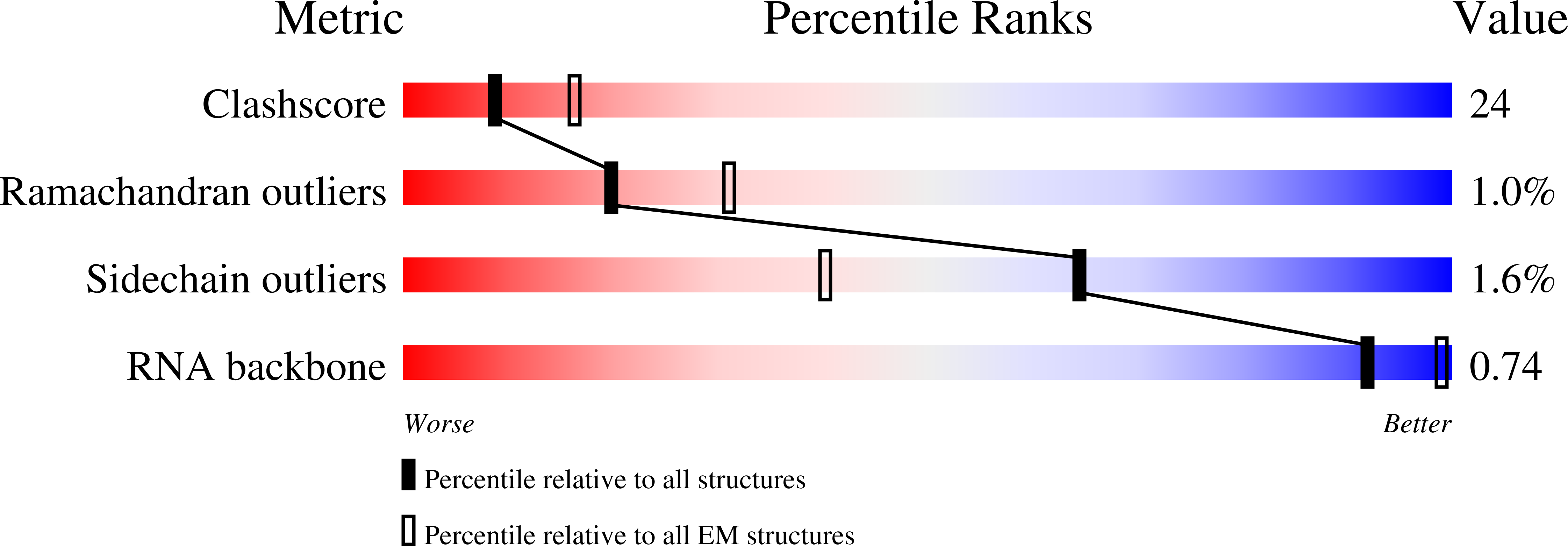Deposition Date
2013-02-13
Release Date
2013-03-06
Last Version Date
2024-05-08
Entry Detail
PDB ID:
3ZN8
Keywords:
Title:
Structural Basis of Signal Sequence Surveillance and Selection by the SRP-SR Complex
Biological Source:
Source Organism(s):
ESCHERICHIA COLI (Taxon ID: 562)
SULFOLOBUS SOLFATARICUS (Taxon ID: 2287)
SACCHAROMYCES CEREVISIAE (Taxon ID: 4932)
SULFOLOBUS SOLFATARICUS (Taxon ID: 2287)
SACCHAROMYCES CEREVISIAE (Taxon ID: 4932)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
12.00 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE