Deposition Date
2013-02-11
Release Date
2014-01-22
Last Version Date
2023-12-20
Entry Detail
PDB ID:
3ZMK
Keywords:
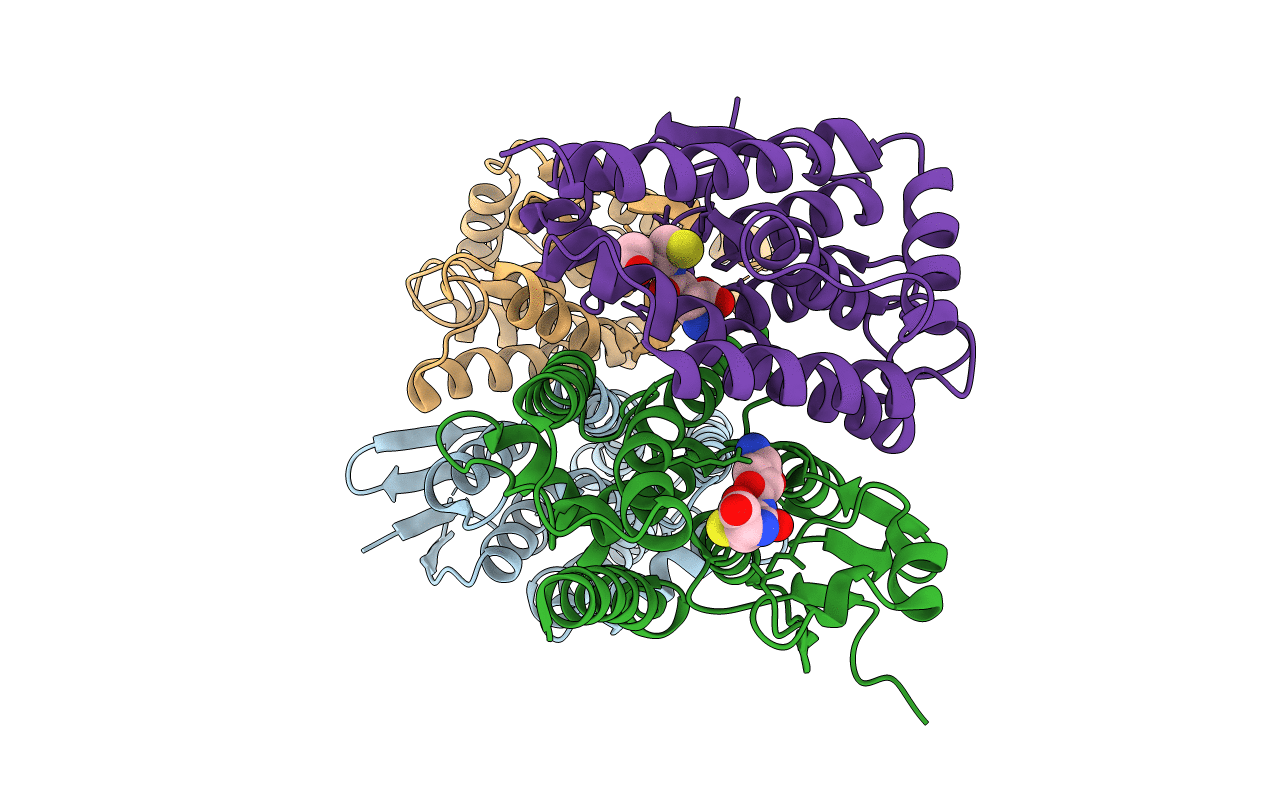
Title:
Anopheles funestus glutathione-s-transferase epsilon 2 (GSTe2) protein structure from different alelles: A single amino acid change confers high level of DDT resistance and cross resistance to permethrin in a major malaria vector in Africa
Biological Source:
Source Organism(s):
ANOPHELES FUNESTUS (Taxon ID: 62324)
Expression System(s):
Method Details:
Experimental Method:
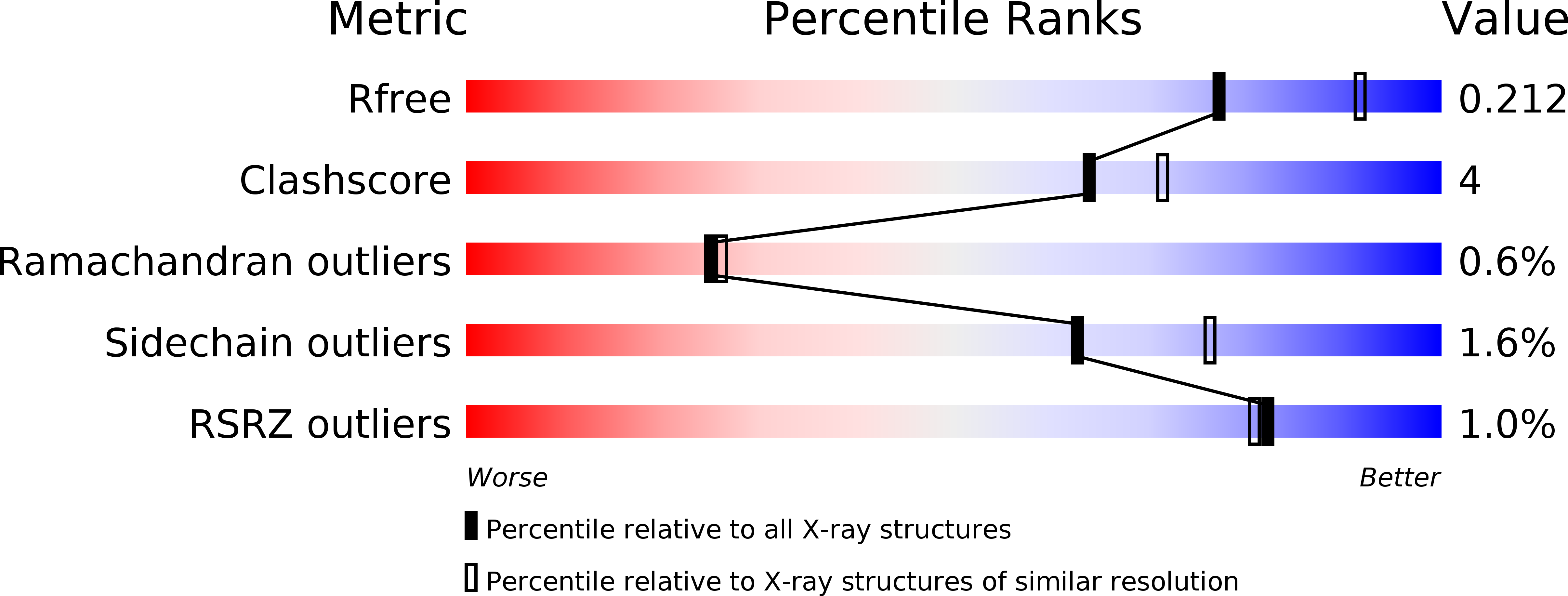
Resolution:
2.20 Å
R-Value Free:
0.20
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
P 1 21 1