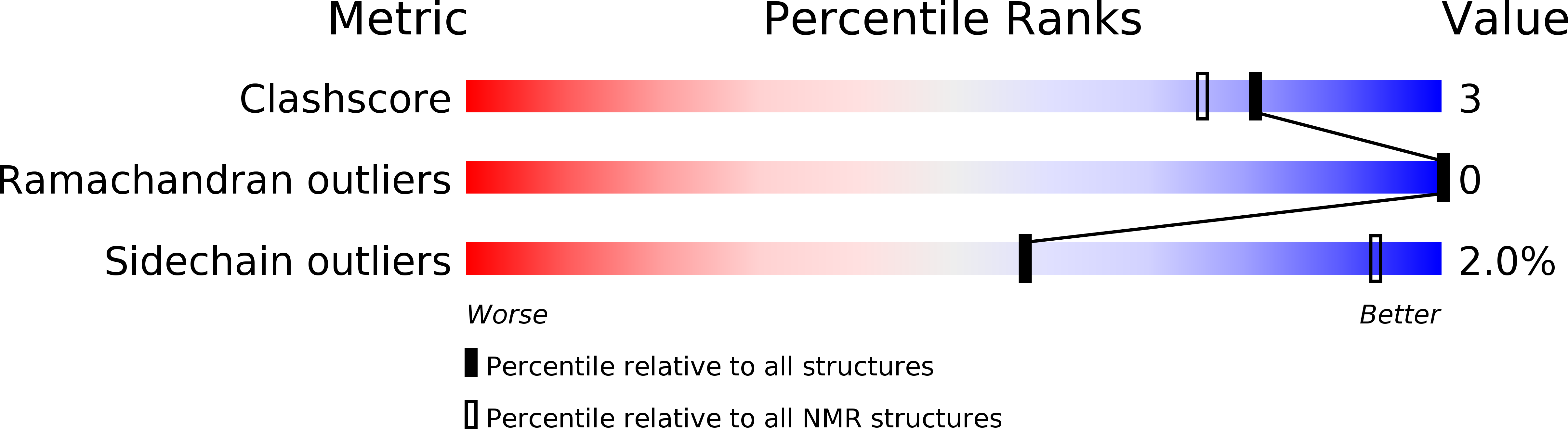
Deposition Date
2013-01-24
Release Date
2013-04-24
Last Version Date
2024-11-13
Entry Detail
PDB ID:
3ZKT
Keywords:
Title:
SOLUTION STRUCTURE OF THE SOMATOSTATIN SST3 RECEPTOR ANTAGONIST TAU- CONOTOXIN CnVA
Biological Source:
Source Organism(s):
CONUS CONSORS (Taxon ID: 101297)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
LEAST RESTRAINT VIOLATION