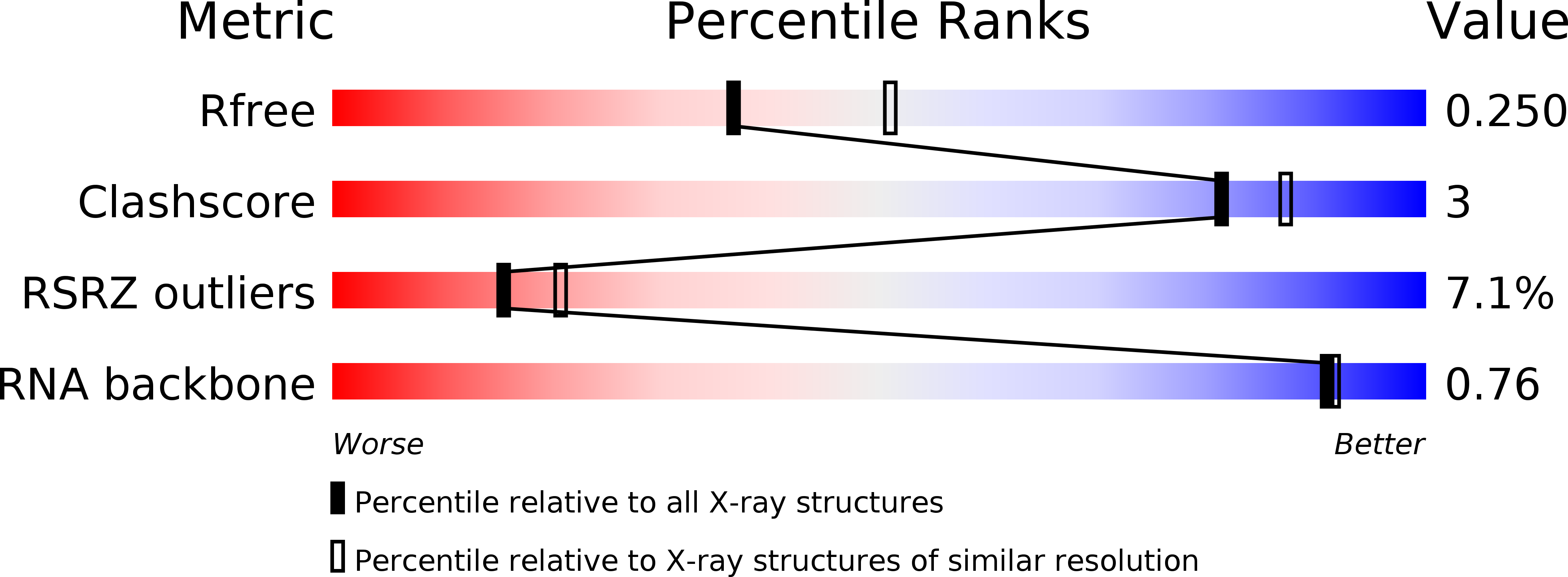Deposition Date
2014-02-27
Release Date
2014-11-05
Last Version Date
2024-03-20
Entry Detail
PDB ID:
3WRU
Keywords:
Title:
Crystal structure of the bacterial ribosomal decoding site in complex with synthetic aminoglycoside with F-HABA group
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Free:
0.26
R-Value Work:
0.24
Space Group:
P 1 21 1