Deposition Date
2014-02-27
Release Date
2015-01-21
Last Version Date
2024-03-20
Entry Detail
PDB ID:
3WRN
Keywords:
Title:
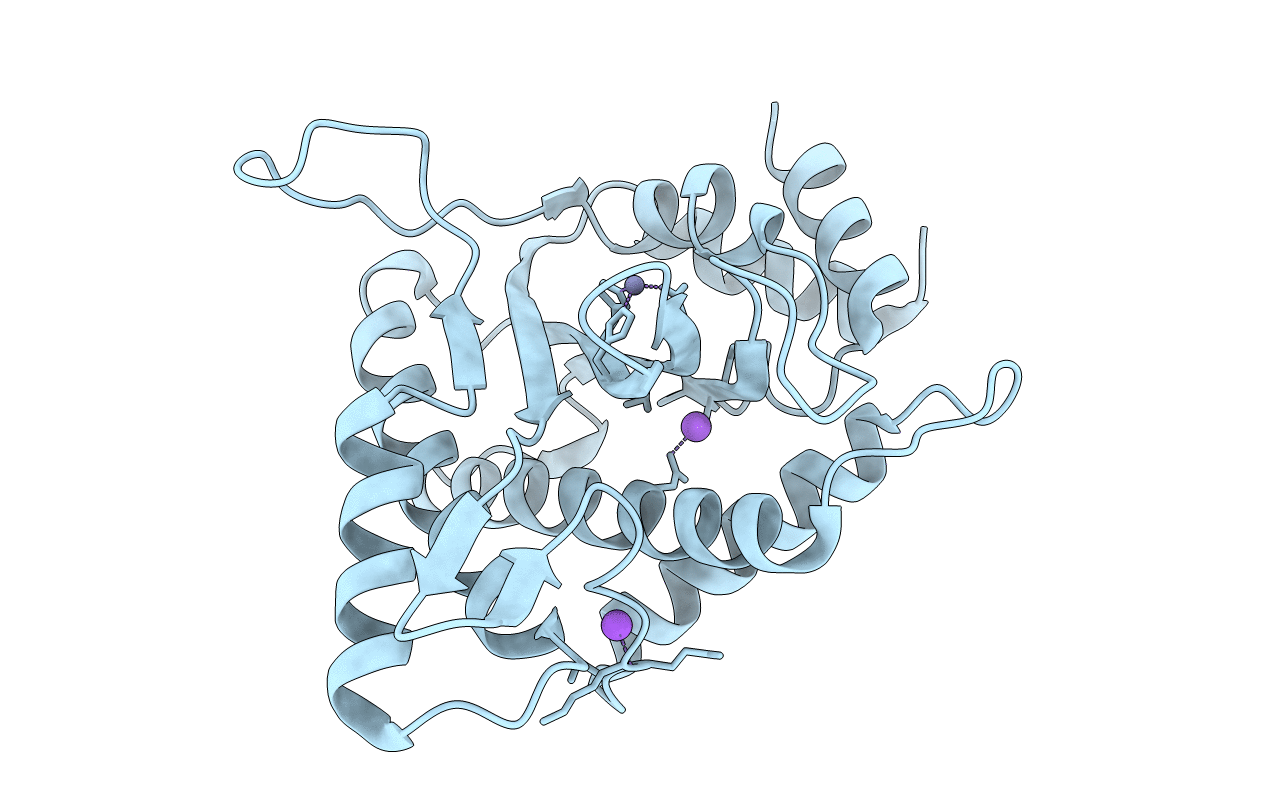
Minute virus of mice non-structural protein-1N-terminal nuclease domain reveals a unique Zn2+ coordination in the active site pocket and shows a novel mode of DNA recognition at the origin of replication
Biological Source:
Source Organism(s):
Murine minute virus (Taxon ID: 648235)
Expression System(s):
Method Details:
Experimental Method:
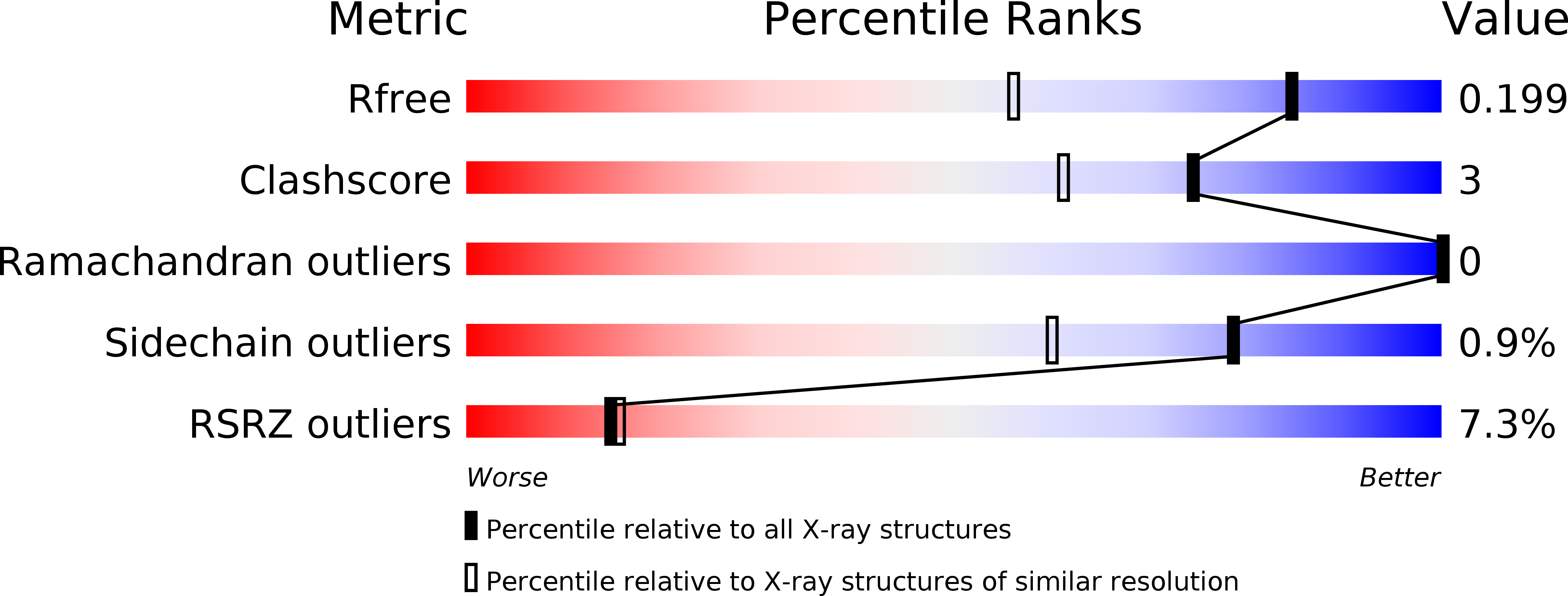
Resolution:
1.52 Å
R-Value Free:
0.19
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 21 21 2