Deposition Date
2013-12-06
Release Date
2014-08-13
Last Version Date
2023-11-08
Entry Detail
PDB ID:
3WN8
Keywords:
Title:
Crystal Structure of Collagen-Model Peptide, (POG)3-PRG-(POG)4
Method Details:
Experimental Method:
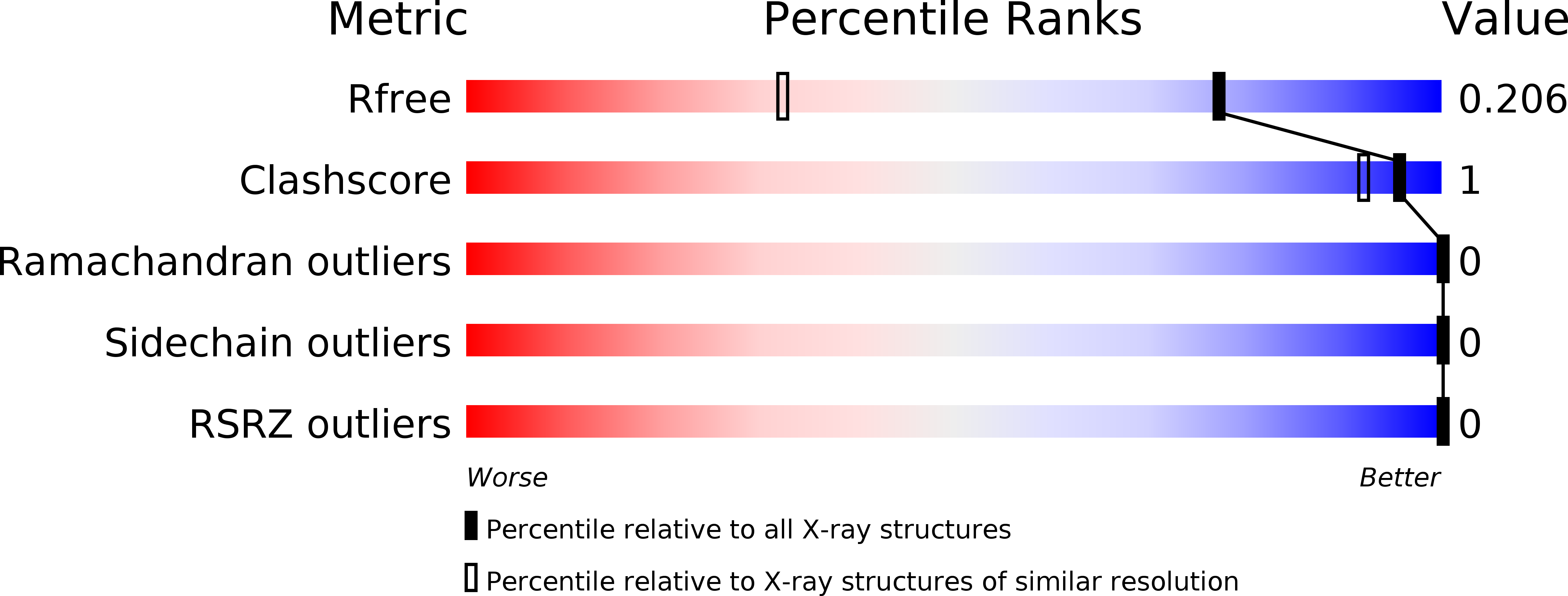
Resolution:
1.45 Å
R-Value Free:
0.20
R-Value Work:
0.14
Space Group:
P 1 21 1