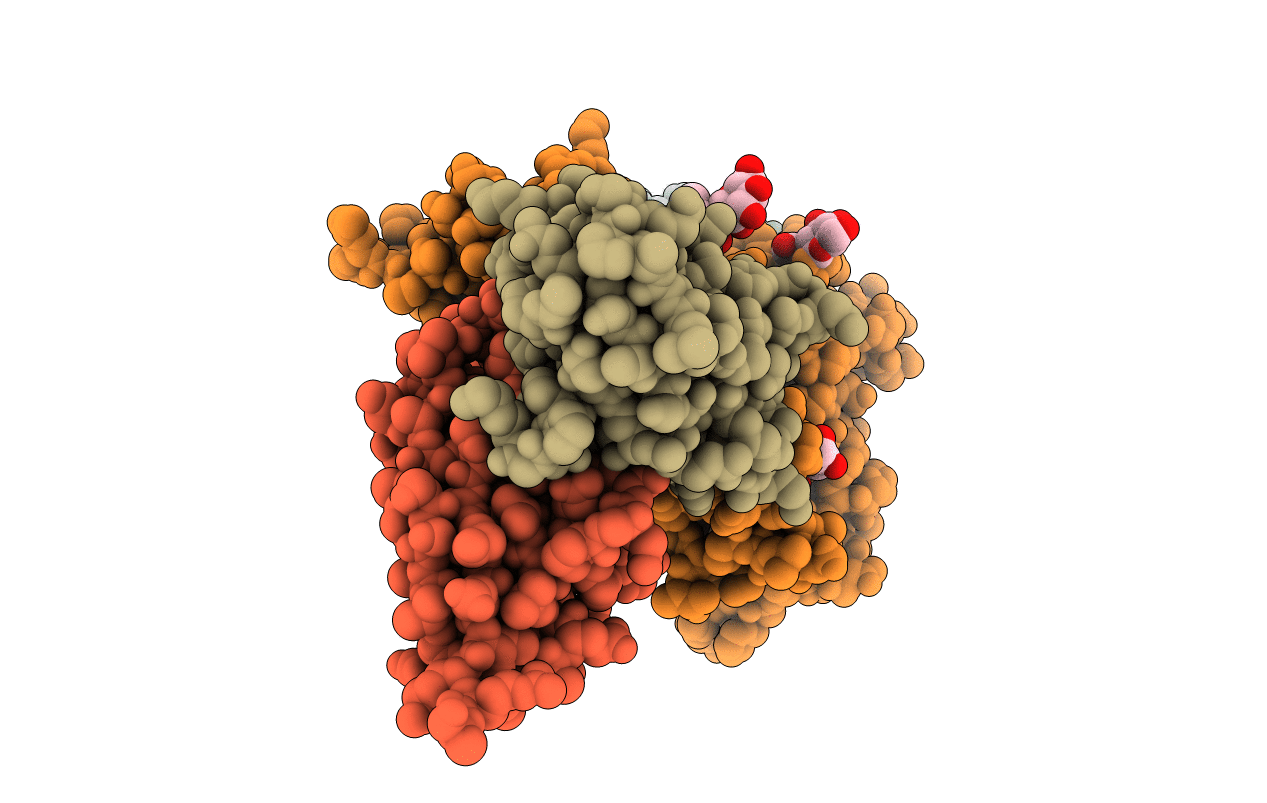
Deposition Date
2012-11-06
Release Date
2013-01-09
Last Version Date
2023-11-08
Entry Detail
PDB ID:
3W12
Keywords:
Title:
Insulin receptor ectodomain construct comprising domains L1-CR in complex with high-affinity insulin analogue [D-PRO-B26]-DTI-NH2, alpha-CT peptide(704-719) and FAB 83-7
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Mus musculus (Taxon ID: 10090)
Mus musculus (Taxon ID: 10090)
Expression System(s):
Method Details:
Experimental Method:
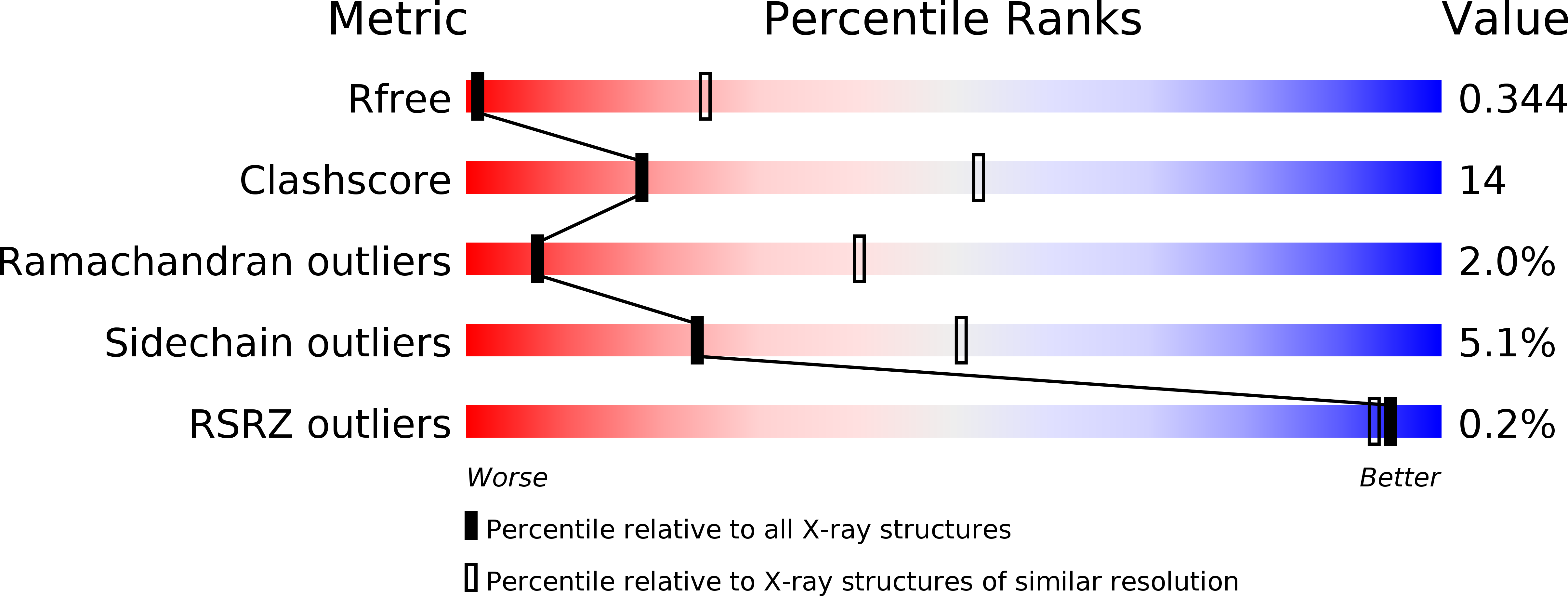
Resolution:
4.30 Å
R-Value Free:
0.34
R-Value Work:
0.28
R-Value Observed:
0.29
Space Group:
P 2 3