Deposition Date
2012-01-09
Release Date
2012-11-21
Last Version Date
2023-09-13
Entry Detail
PDB ID:
3VF7
Keywords:
Title:
Crystal Structure of HIV-1 Protease Mutant L76V with novel P1'-Ligands GRL-02031
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
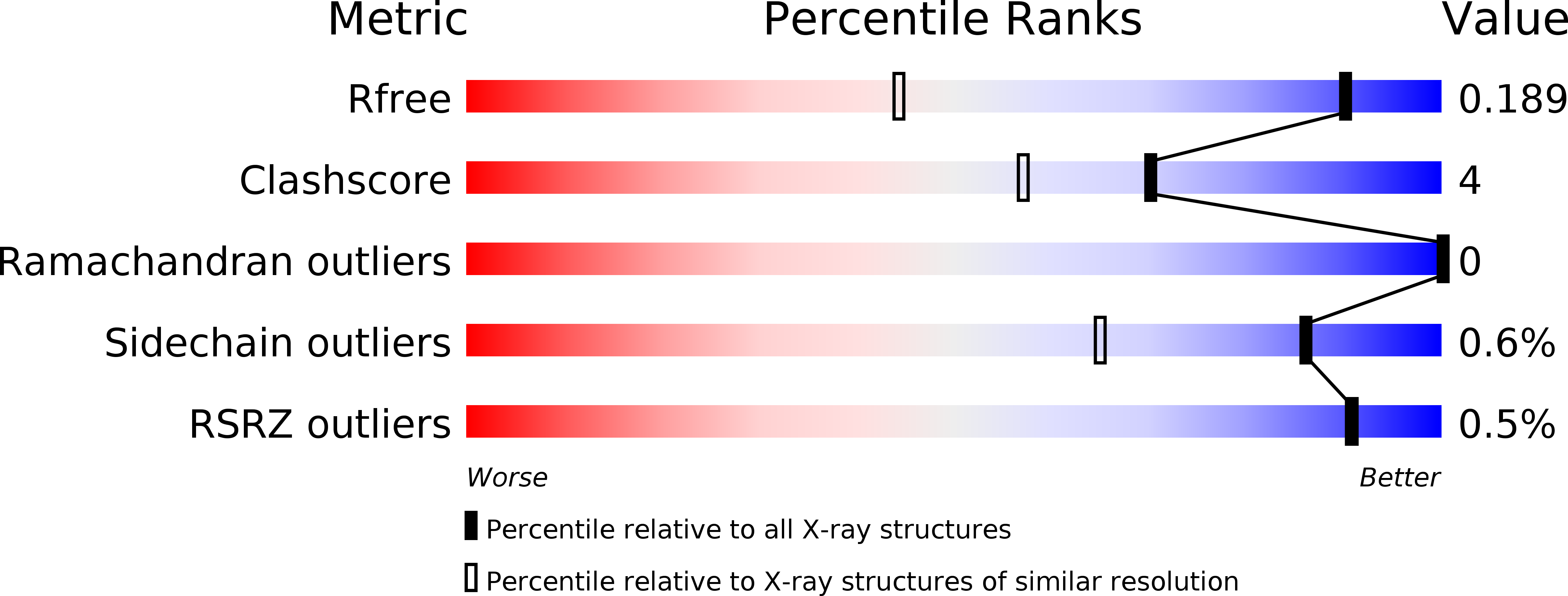
Resolution:
1.30 Å
R-Value Free:
0.19
R-Value Observed:
0.15
Space Group:
P 21 21 2