Deposition Date
2011-12-14
Release Date
2012-01-04
Last Version Date
2023-09-13
Entry Detail
PDB ID:
3V45
Keywords:
Title:
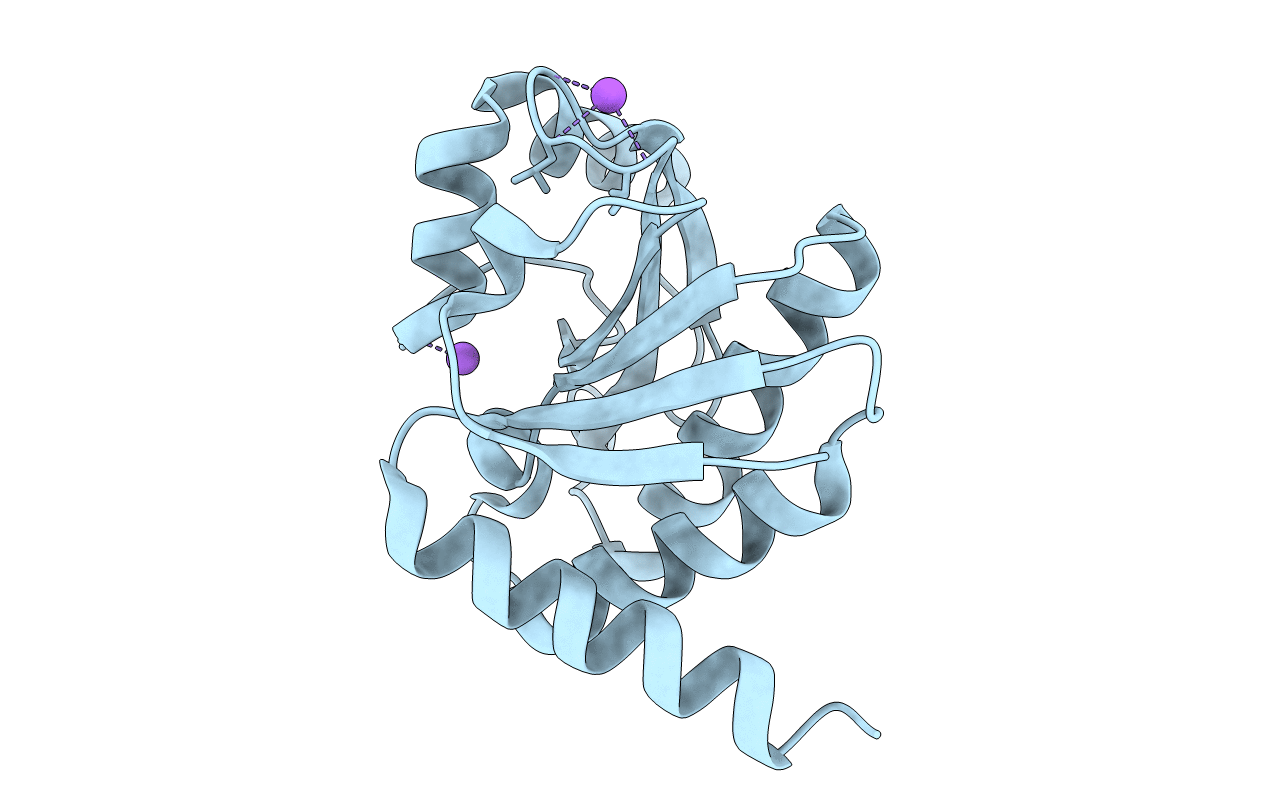
Crystal Structure of de novo designed serine hydrolase OSH55, Northeast Structural Genomics Consortium Target OR130
Method Details:
Experimental Method:
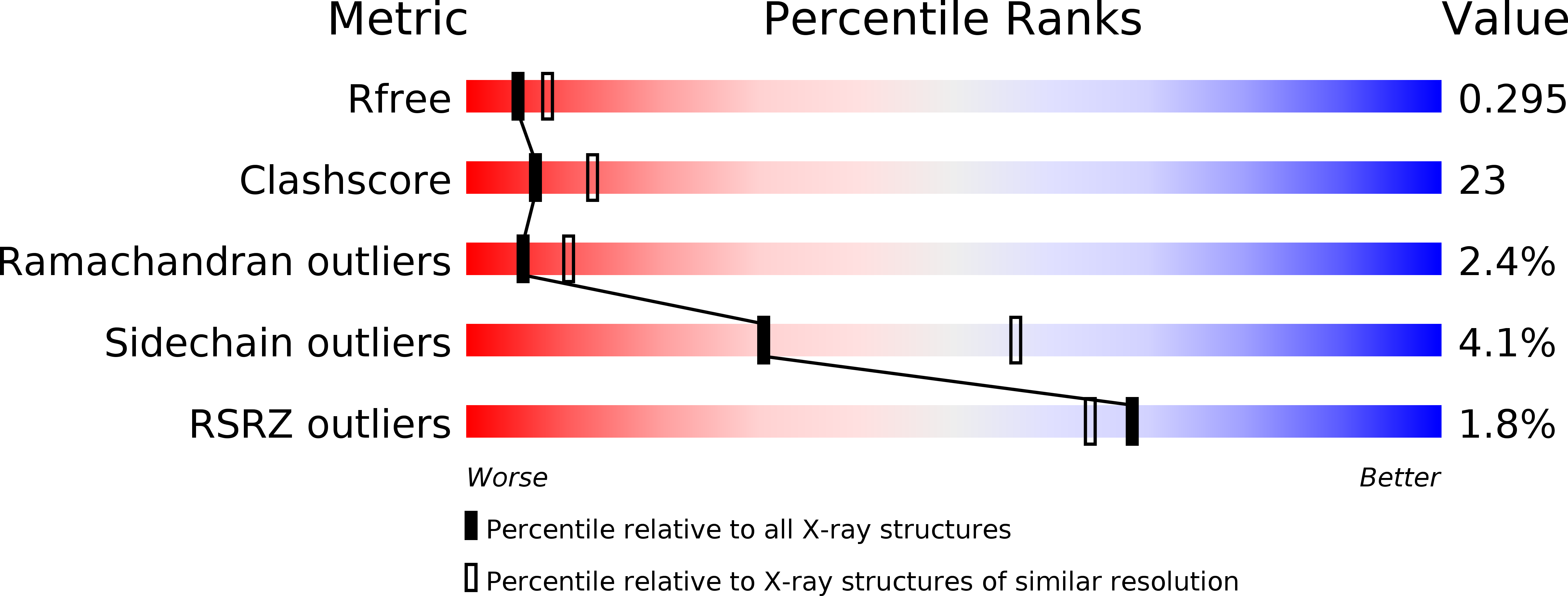
Resolution:
2.60 Å
R-Value Free:
0.28
R-Value Work:
0.20
Space Group:
P 2 2 21