Deposition Date
2011-12-10
Release Date
2012-02-29
Last Version Date
2024-10-30
Entry Detail
PDB ID:
3V1S
Keywords:
Title:
Scaffold tailoring by a newly detected Pictet-Spenglerase ac-tivity of strictosidine synthase (STR1): from the common tryp-toline skeleton to the rare piperazino-indole framework
Biological Source:
Source Organism(s):
Rauvolfia serpentina (Taxon ID: 4060)
Expression System(s):
Method Details:
Experimental Method:
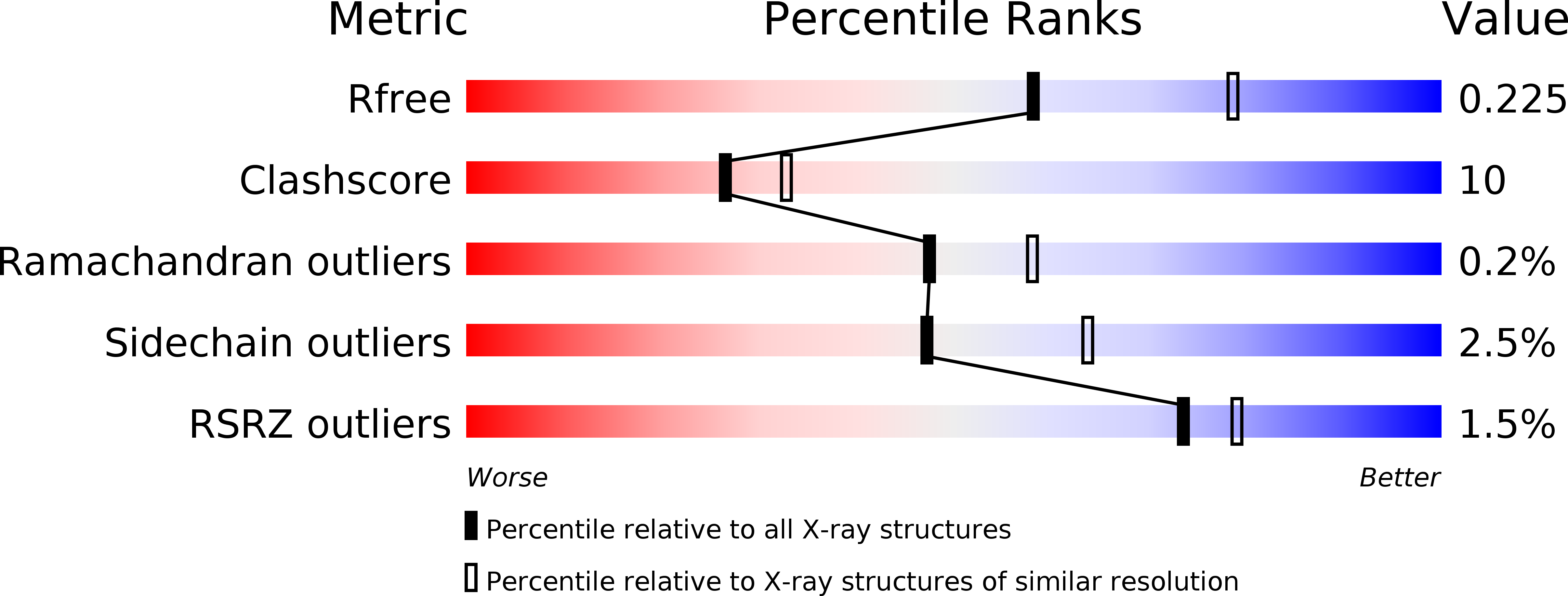
Resolution:
2.33 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
H 3