Deposition Date
2011-10-20
Release Date
2012-01-25
Last Version Date
2023-09-13
Entry Detail
PDB ID:
3U9Z
Keywords:
Title:
Crystal structure between actin and a protein construct containing the first beta-thymosin domain of drosophila ciboulot (residues 2-58) with the three mutations N26D/Q27K/D28S
Biological Source:
Source Organism(s):
Drosophila melanogaster (Taxon ID: 7227)
Oryctolagus cuniculus (Taxon ID: 9986)
Oryctolagus cuniculus (Taxon ID: 9986)
Expression System(s):
Method Details:
Experimental Method:
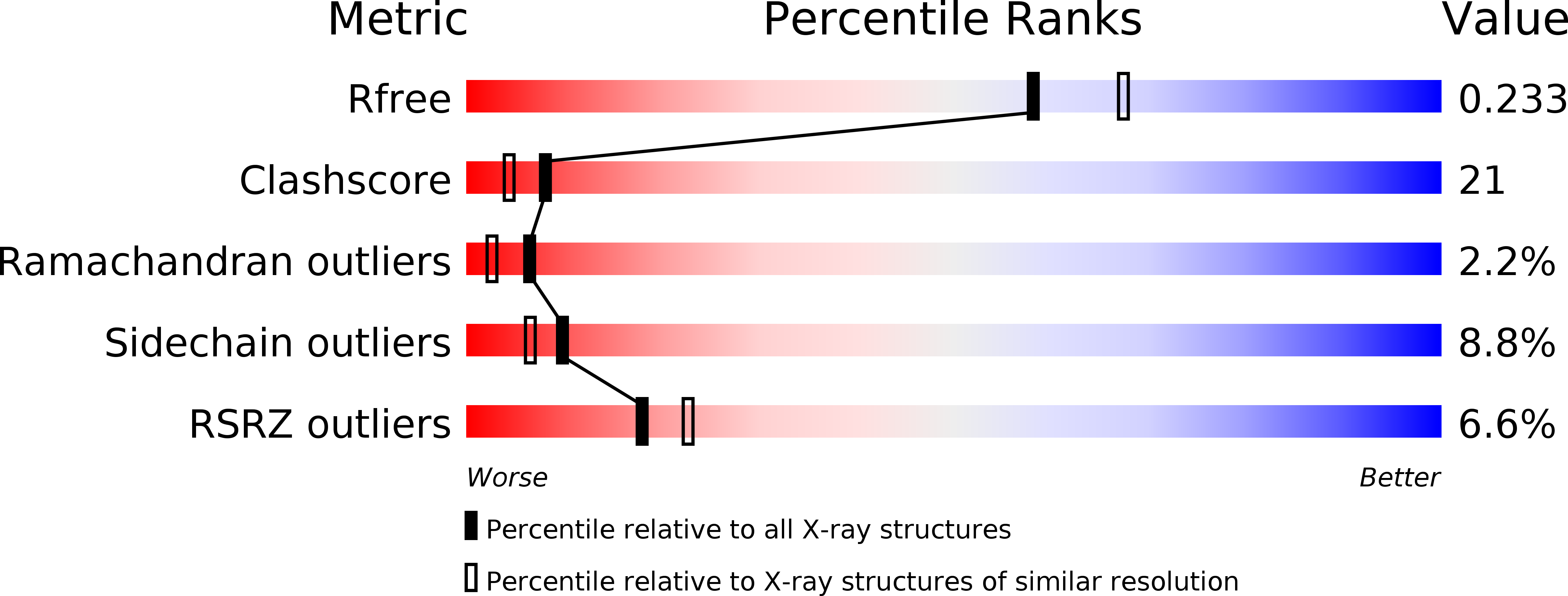
Resolution:
2.09 Å
R-Value Free:
0.22
R-Value Work:
0.15
R-Value Observed:
0.16
Space Group:
P 21 21 21