Deposition Date
2011-10-13
Release Date
2012-07-18
Last Version Date
2024-11-06
Entry Detail
PDB ID:
3U7B
Keywords:
Title:
A new crystal structure of a Fusarium oxysporum GH10 xylanase reveals the presence of an extended loop on top of the catalytic cleft
Biological Source:
Source Organism(s):
Fusarium oxysporum (Taxon ID: 5507)
Method Details:
Experimental Method:
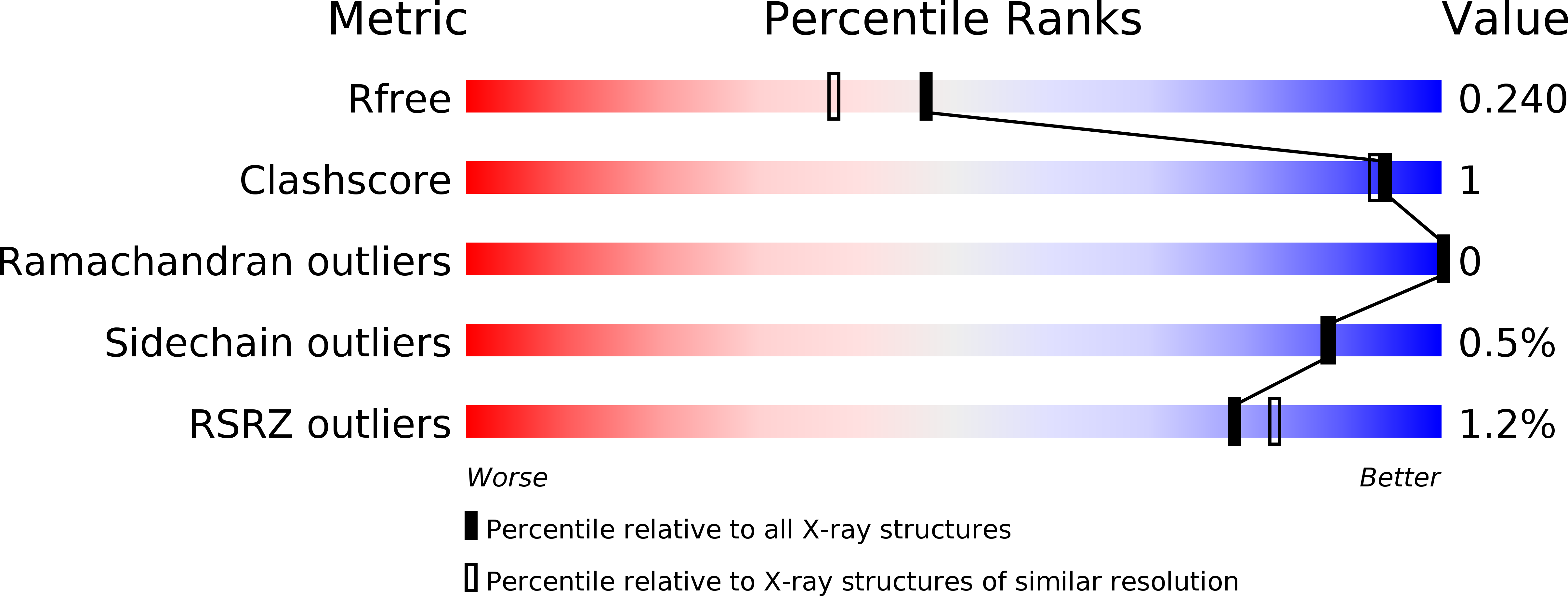
Resolution:
1.94 Å
R-Value Free:
0.24
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 41 21 2