Deposition Date
2011-10-05
Release Date
2012-03-07
Last Version Date
2023-11-01
Entry Detail
PDB ID:
3U3G
Keywords:
Title:
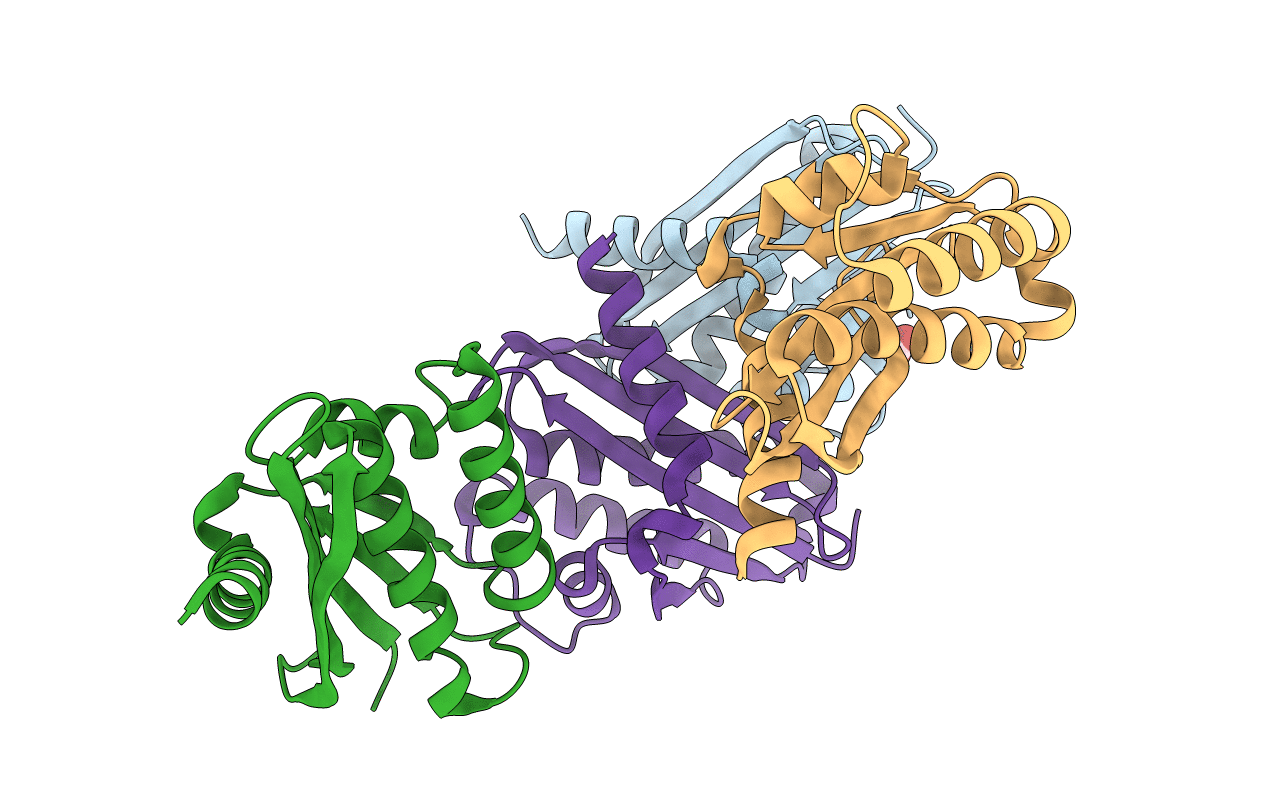
Structure of LC11-RNase H1 Isolated from Compost by Metagenomic Approach: Insight into the Structural Bases for Unusual Enzymatic Properties of Sto-RNase H1
Biological Source:
Source Organism(s):
uncultured organism (Taxon ID: 155900)
Expression System(s):
Method Details:
Experimental Method:
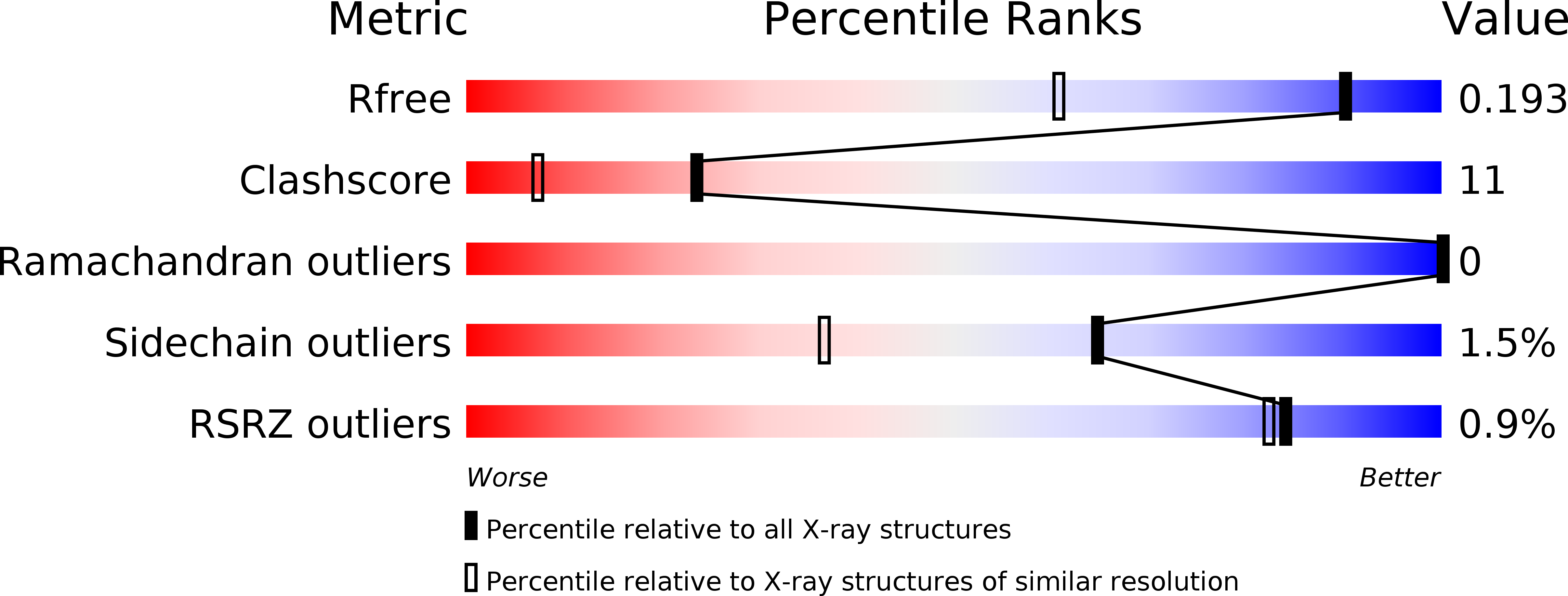
Resolution:
1.40 Å
R-Value Free:
0.19
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 1 21 1