Deposition Date
2011-09-16
Release Date
2012-08-29
Last Version Date
2024-02-28
Entry Detail
PDB ID:
3TUD
Keywords:
Title:
Crystal structure of SYK kinase domain with N-(4-methyl-3-(8-methyl-7-oxo-2-(phenylamino)-7,8-dihydropyrido[2,3-d]pyrimidin-6-yl)phenyl)-3-(trifluoromethyl)benzamide
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
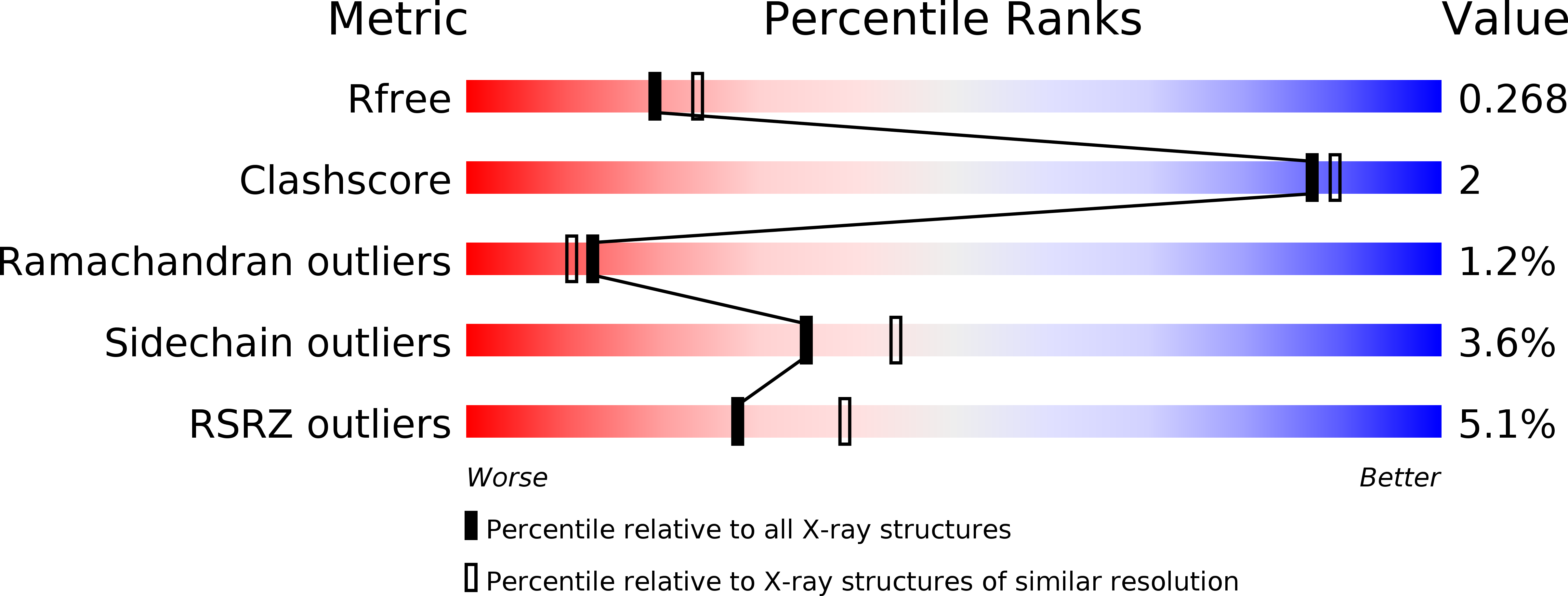
Resolution:
2.33 Å
R-Value Free:
0.26
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21