Deposition Date
2011-07-22
Release Date
2012-01-25
Last Version Date
2023-09-13
Entry Detail
PDB ID:
3T1Y
Keywords:
Title:
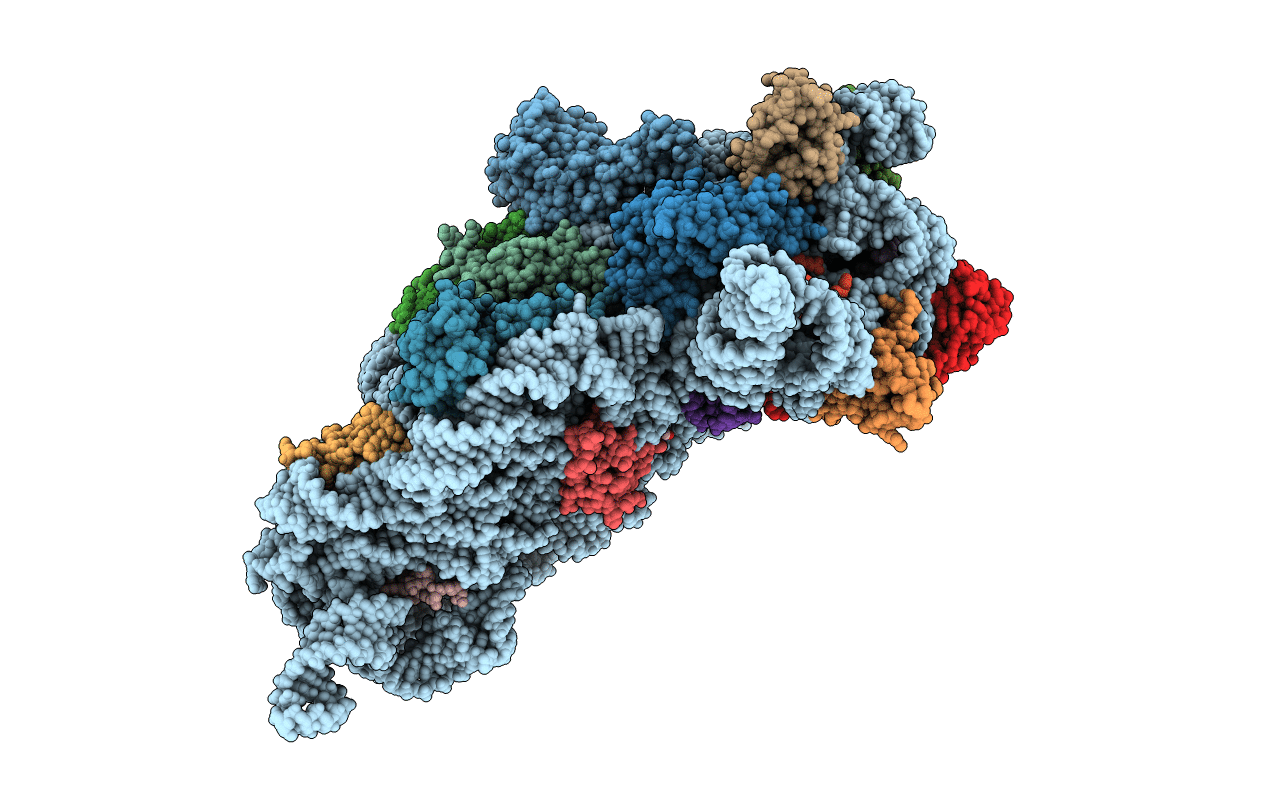
Structure of the Thermus thermophilus 30S ribosomal subunit complexed with a human anti-codon stem loop (HASL) of transfer RNA Lysine 3 (TRNALYS3) bound to an mRNA with an AAG-codon in the A-site and paromomycin
Biological Source:
Source Organism(s):
Thermus thermophilus (Taxon ID: 300852)
Thermus thermophilus (Taxon ID: 262724)
Thermus thermophilus (Taxon ID: 274)
Thermus thermophilus (Taxon ID: 262724)
Thermus thermophilus (Taxon ID: 274)
Method Details:
Experimental Method:
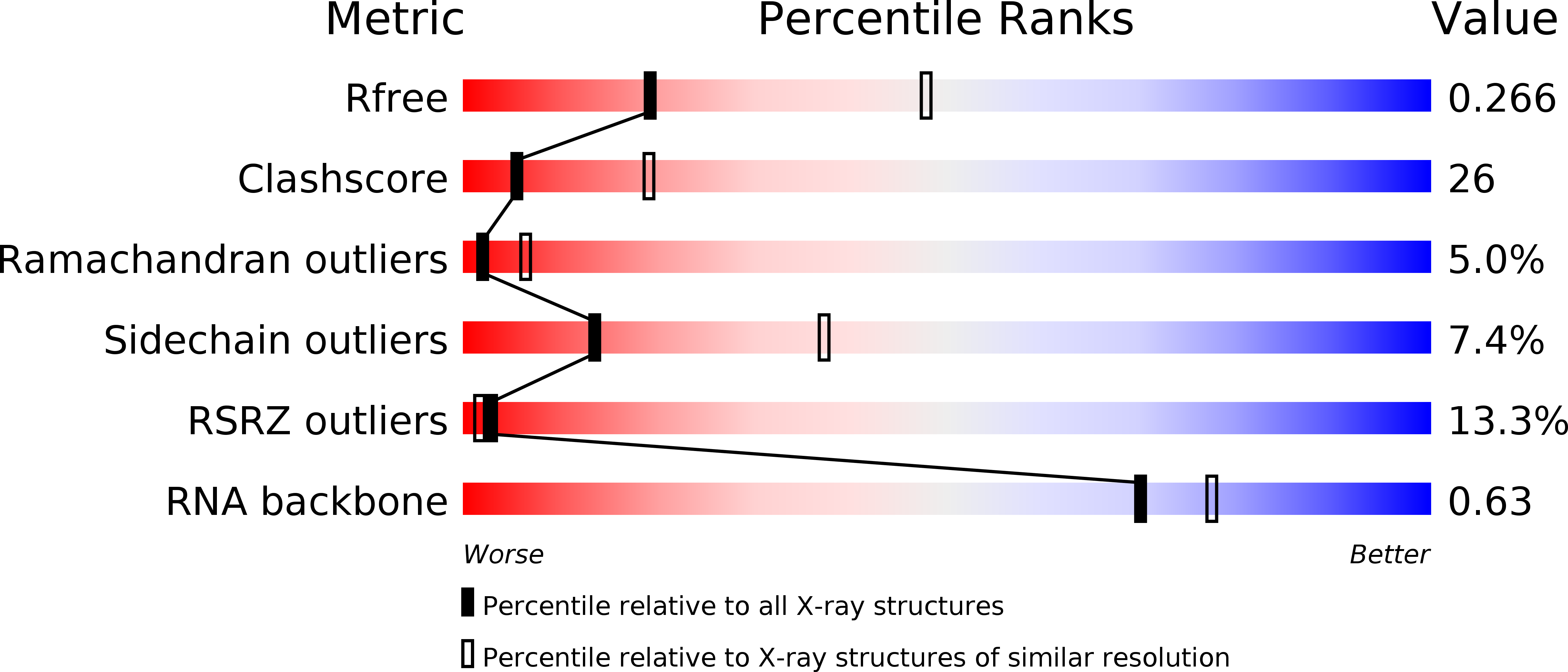
Resolution:
2.80 Å
R-Value Free:
0.26
R-Value Work:
0.24
Space Group:
P 41 21 2