Deposition Date
2011-06-24
Release Date
2011-07-13
Last Version Date
2023-09-13
Entry Detail
PDB ID:
3SLD
Keywords:
Title:
Structural characterization of a GII.4 2004 norovirus variant (TCH05) bound to A trisaccharide
Biological Source:
Source Organism(s):
Norovirus Hu/GII.4/2004/NL (Taxon ID: 311380)
Expression System(s):
Method Details:
Experimental Method:
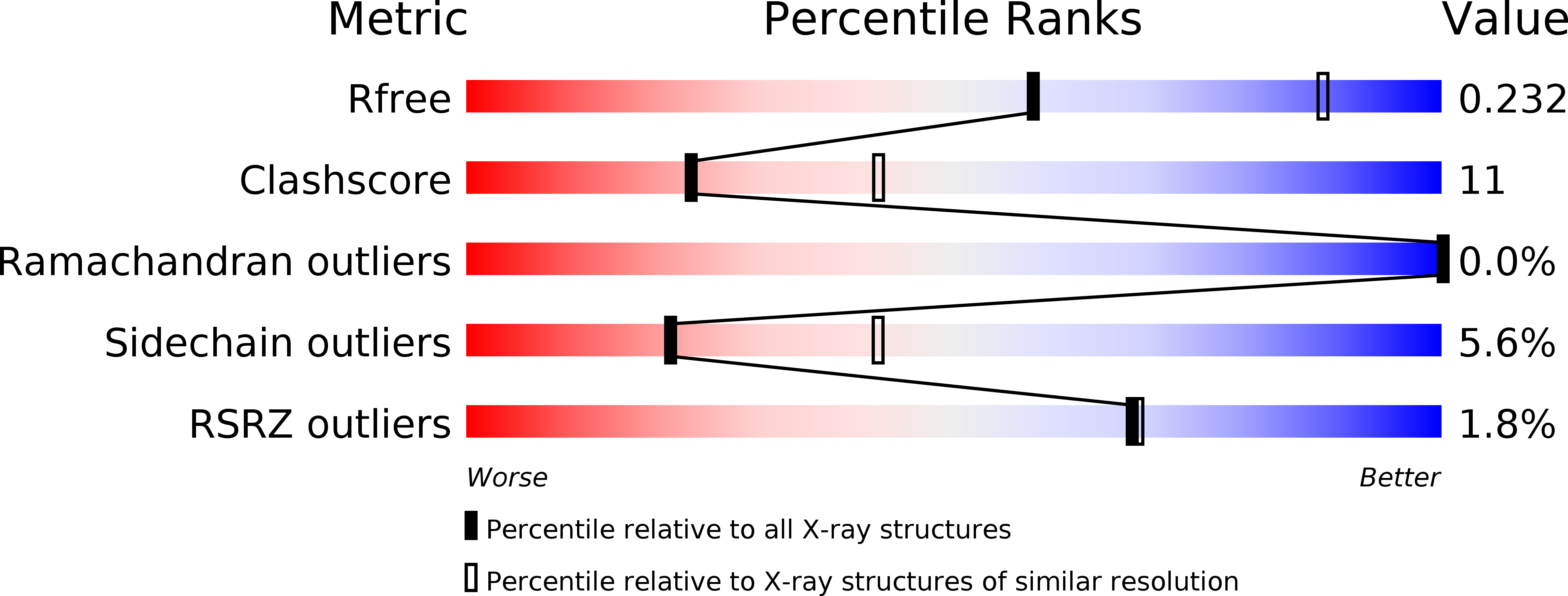
Resolution:
2.68 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
C 2 2 21