Deposition Date
2011-06-21
Release Date
2012-01-25
Last Version Date
2023-09-13
Entry Detail
PDB ID:
3SJH
Keywords:
Title:
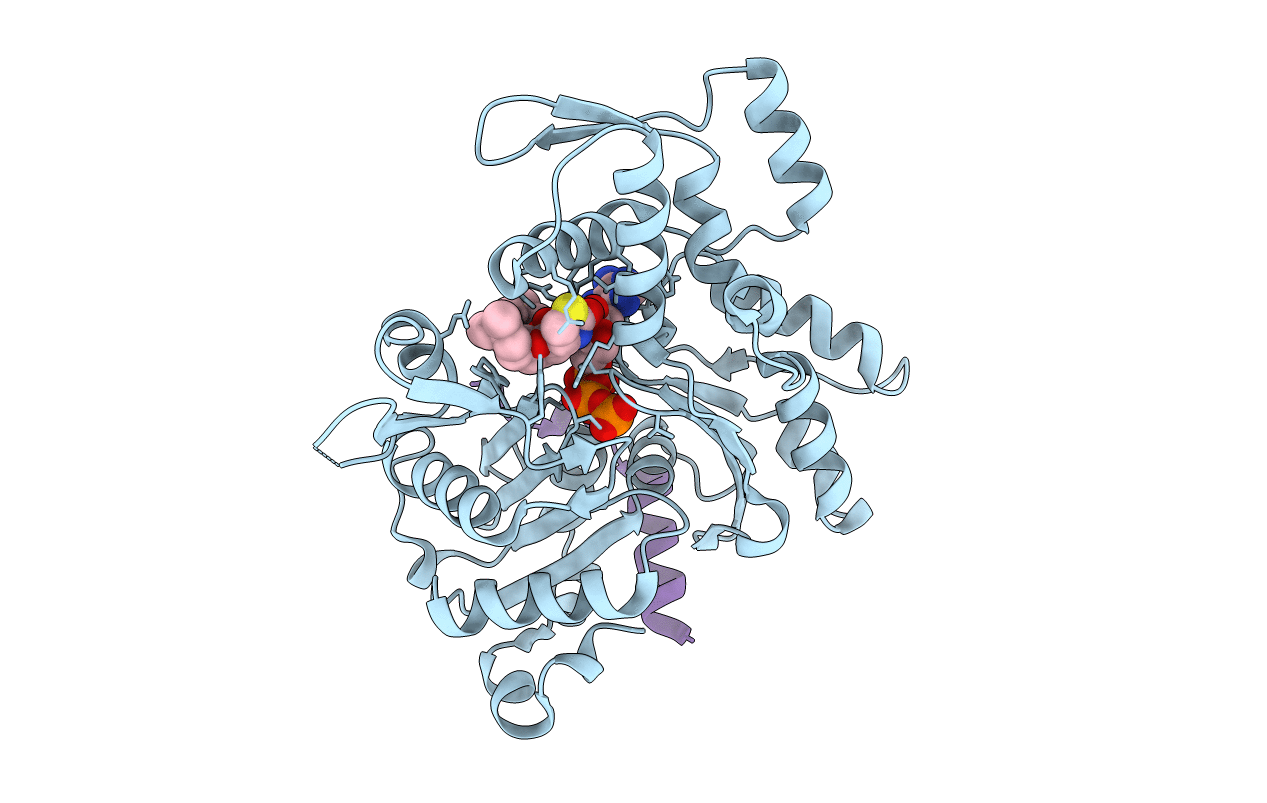
Crystal Structure of a chimera containing the N-terminal domain (residues 8-29) of drosophila Ciboulot and the C-terminal domain (residues 18-44) of bovine Thymosin-beta4, bound to G-actin-ATP-Latrunculin A
Biological Source:
Source Organism(s):
Drosophila melanogaster (Taxon ID: 7227)
Bos taurus (Taxon ID: 9913)
Oryctolagus cuniculus (Taxon ID: 9986)
Bos taurus (Taxon ID: 9913)
Oryctolagus cuniculus (Taxon ID: 9986)
Expression System(s):
Method Details:
Experimental Method:
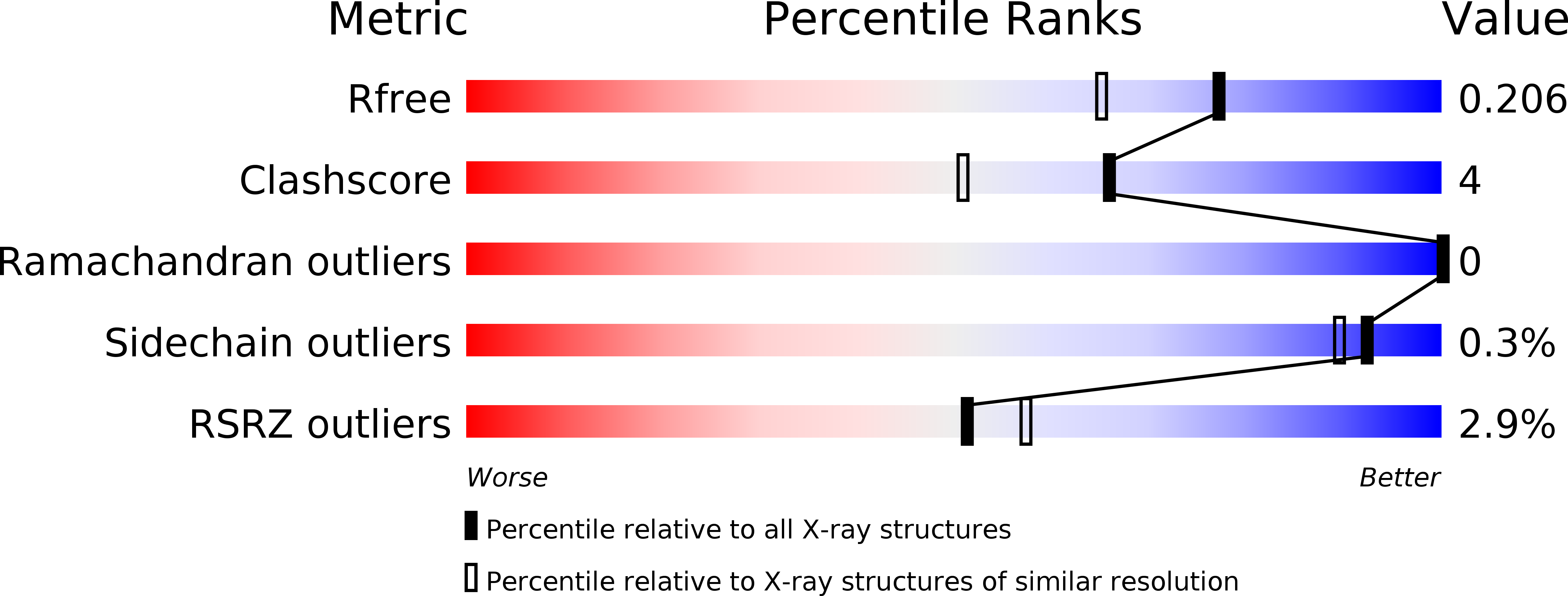
Resolution:
1.75 Å
R-Value Free:
0.19
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
P 21 21 21