Deposition Date
2011-06-01
Release Date
2012-06-06
Last Version Date
2023-09-13
Entry Detail
PDB ID:
3S9O
Keywords:
Title:
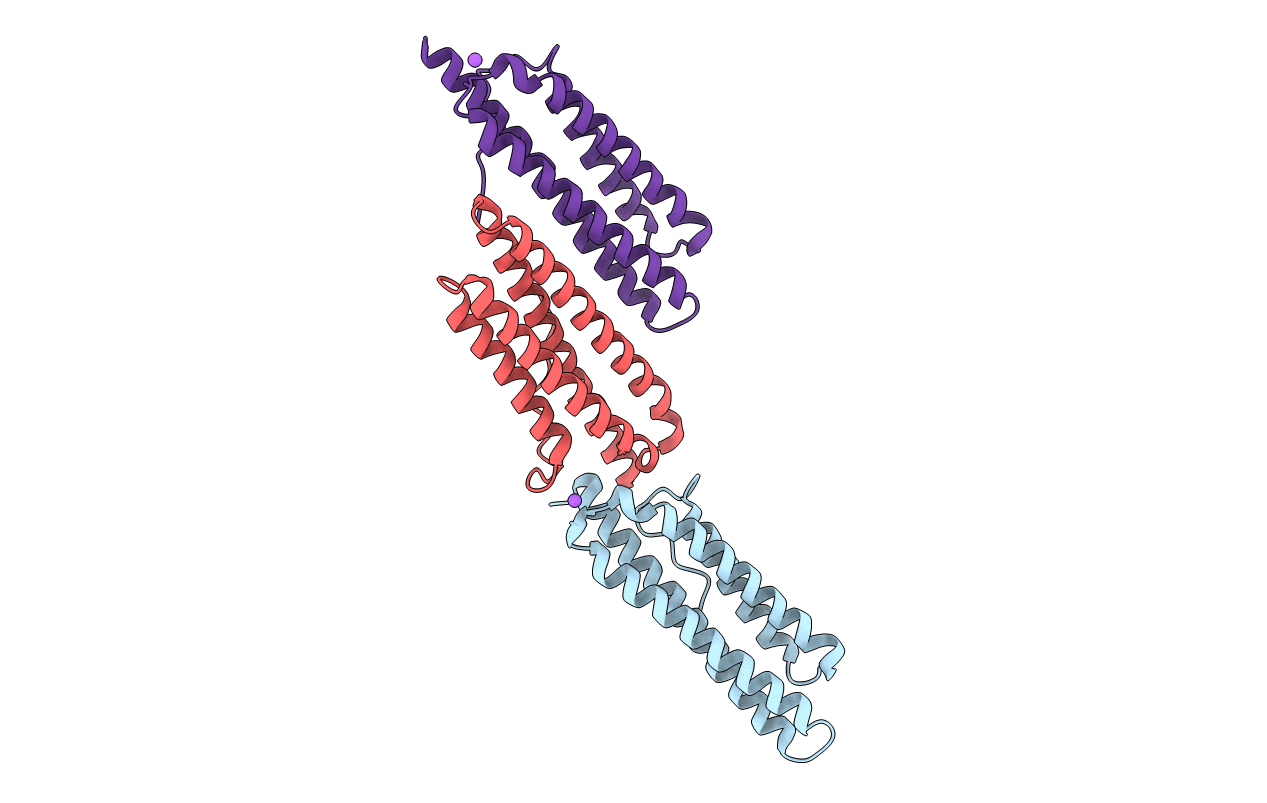
The Focal Adhesion Targeting (FAT) domain of the Focal Adhesion Kinase showing N-terminal interactions in cis
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
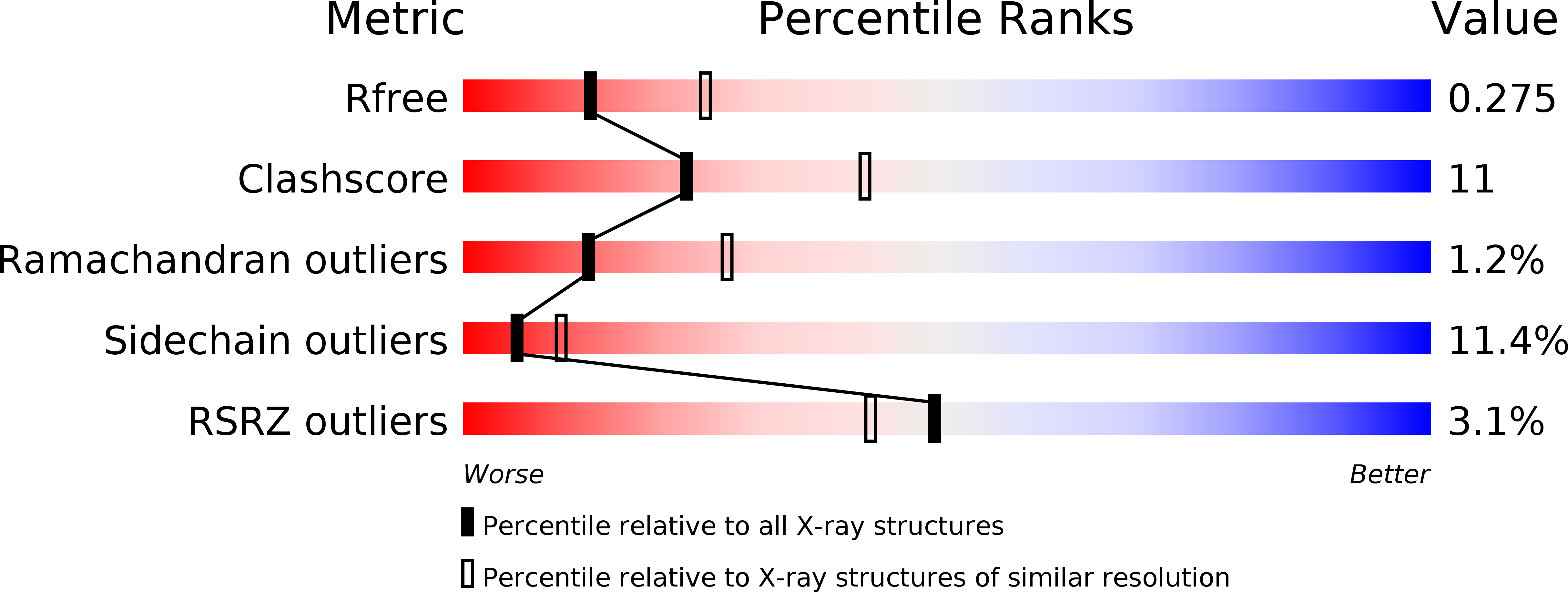
Resolution:
2.60 Å
R-Value Free:
0.28
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
C 2 2 21