Deposition Date
2011-02-22
Release Date
2011-03-09
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3QT9
Keywords:
Title:
Analysis of a new family of widely distributed metal-independent alpha mannosidases provides unique insight into the processing of N-linked glycans, Clostridium perfringens CPE0426 complexed with alpha-1,6-linked 1-thio-alpha-mannobiose
Biological Source:
Source Organism(s):
Clostridium perfringens (Taxon ID: 1502)
Expression System(s):
Method Details:
Experimental Method:
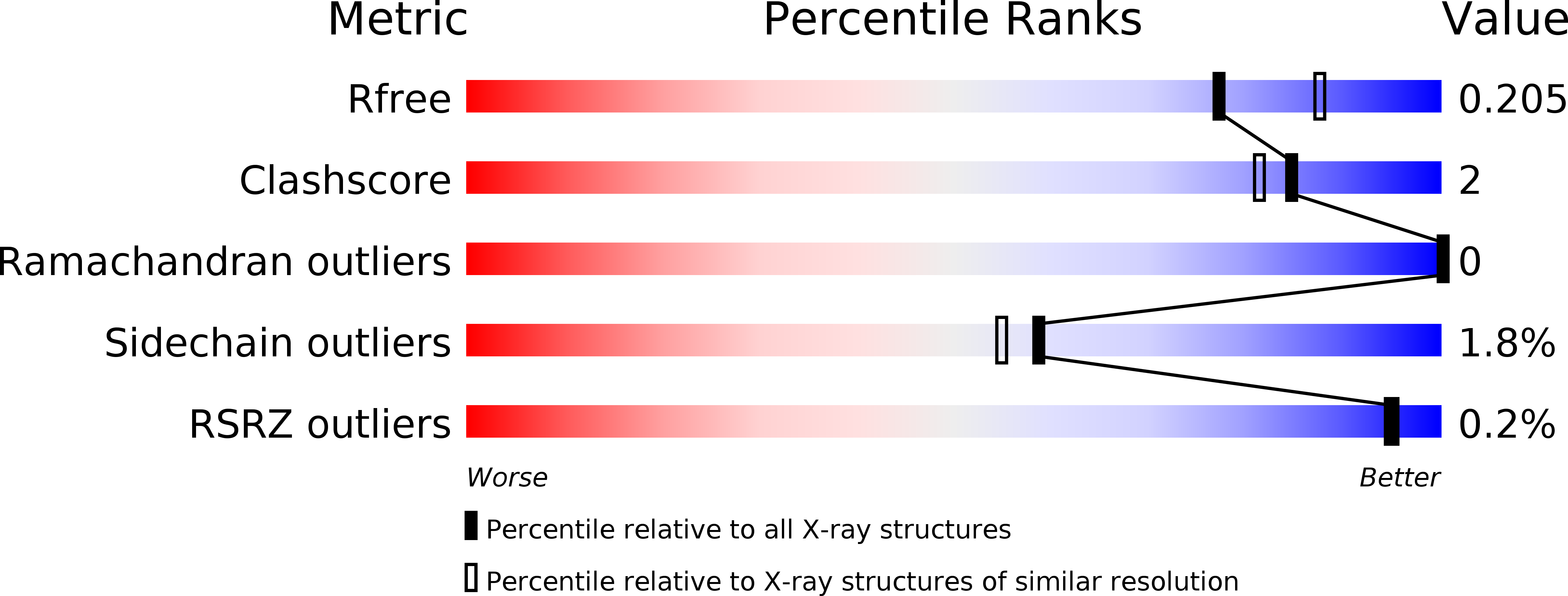
Resolution:
2.05 Å
R-Value Free:
0.20
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 21 21 21