Deposition Date
2011-02-04
Release Date
2011-08-03
Last Version Date
2024-12-25
Entry Detail
PDB ID:
3QMO
Keywords:
Title:
X-ray crystal structure of NS-398 bound to the cyclooxygenase channel of cyclooxygenase-2
Biological Source:
Source Organism:
Mus musculus (Taxon ID: 10090)
Host Organism:
Method Details:
Experimental Method:
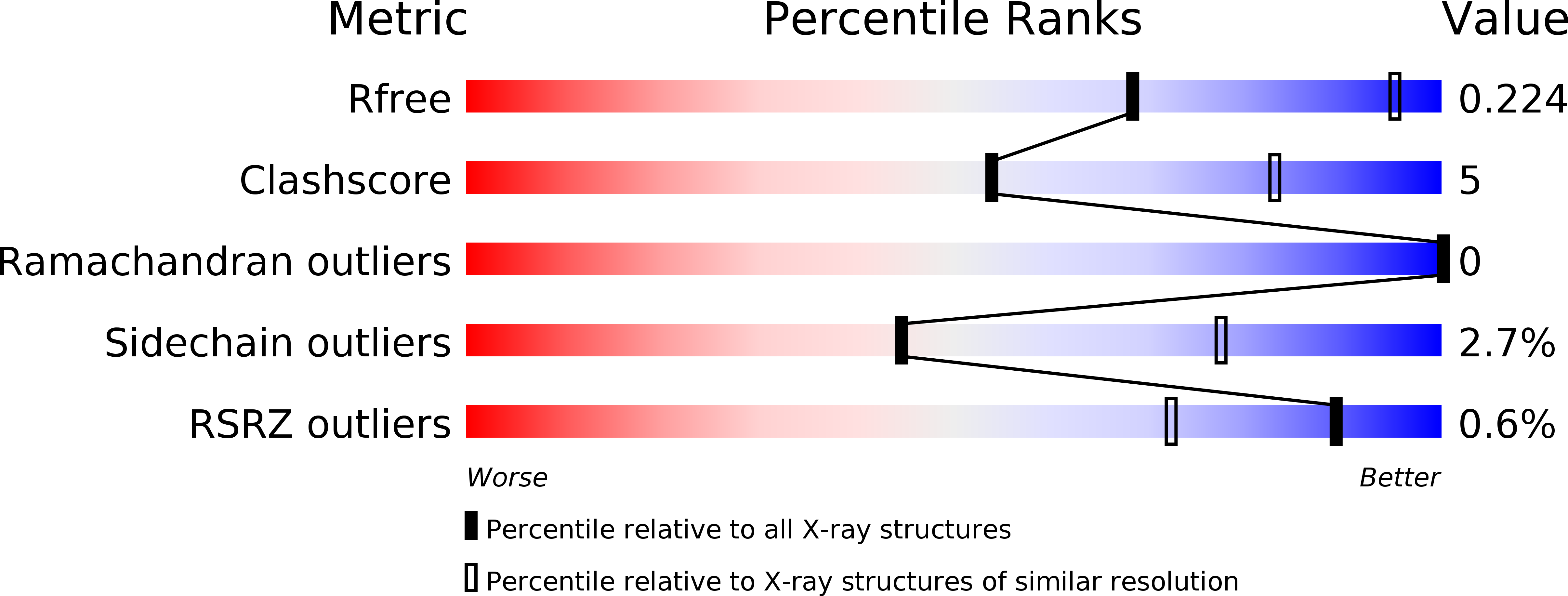
Resolution:
3.00 Å
R-Value Free:
0.22
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
I 2 2 2