Deposition Date
2011-02-03
Release Date
2011-08-31
Last Version Date
2023-09-13
Entry Detail
PDB ID:
3QM1
Keywords:
Title:
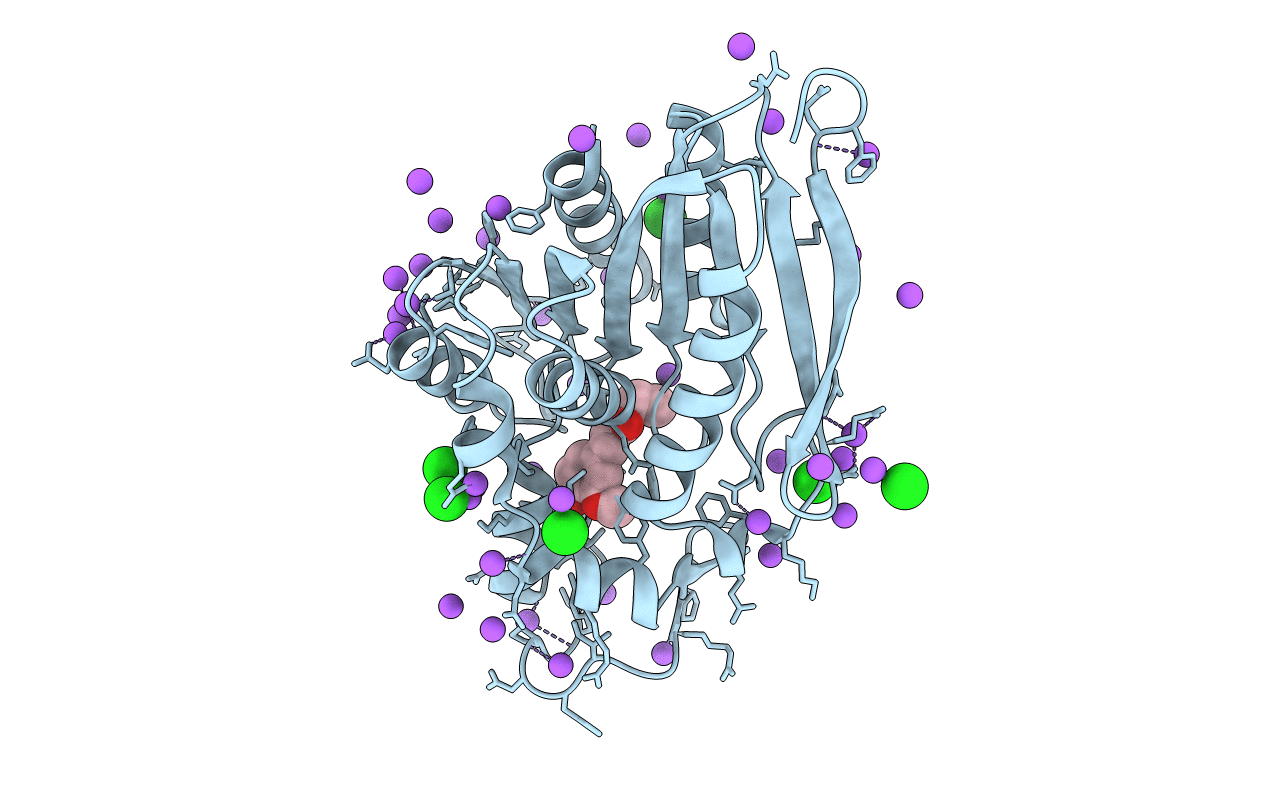
CRYSTAL STRUCTURE OF THE LACTOBACILLUS JOHNSONII CINNAMOYL ESTERASE LJ0536 S106A MUTANT IN COMPLEX WITH ETHYLFERULATE, Form II
Biological Source:
Source Organism(s):
Lactobacillus johnsonii (Taxon ID: 33959)
Expression System(s):
Method Details:
Experimental Method:
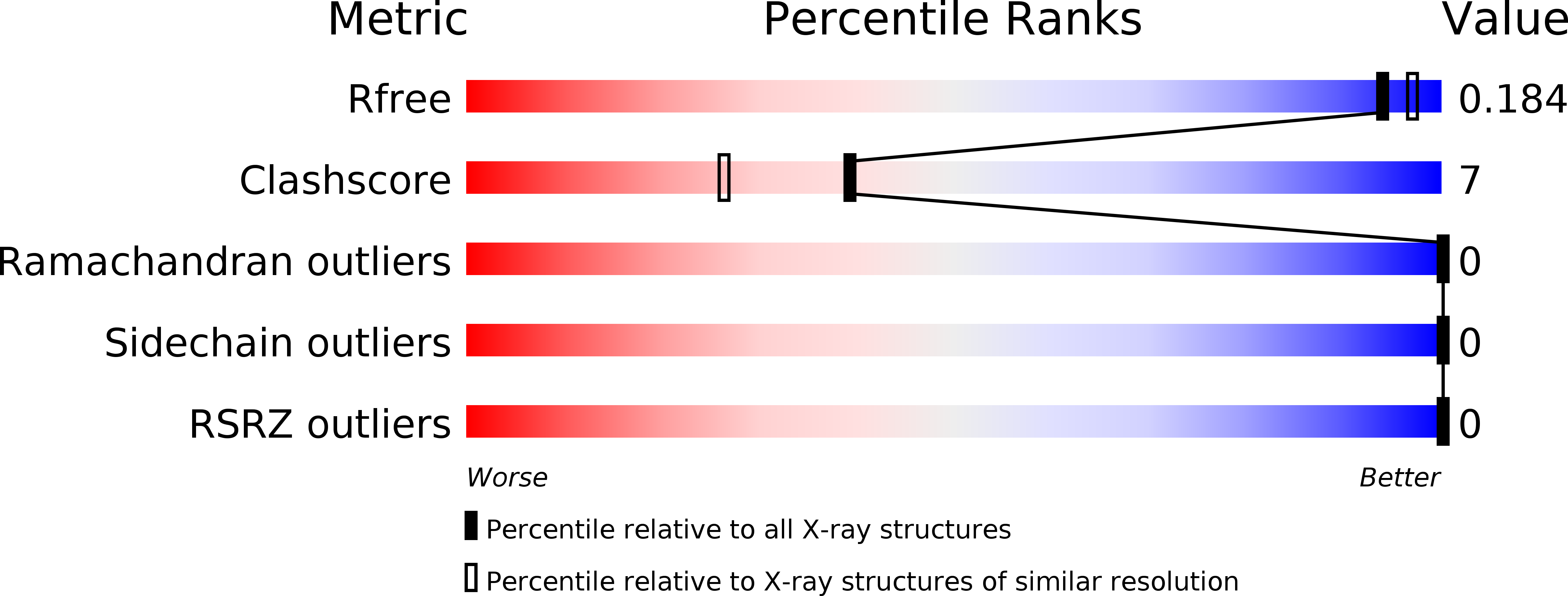
Resolution:
1.82 Å
R-Value Free:
0.19
R-Value Work:
0.14
R-Value Observed:
0.14
Space Group:
C 2 2 21