Deposition Date
2011-01-26
Release Date
2011-07-20
Last Version Date
2023-09-13
Entry Detail
PDB ID:
3QHY
Keywords:
Title:
Structural, thermodynamic and kinetic analysis of the picomolar binding affinity interaction of the beta-lactamase inhibitor protein-II (BLIP-II) with class A beta-lactamases
Biological Source:
Source Organism(s):
Bacillus anthracis (Taxon ID: 1392)
Streptomyces exfoliatus (Taxon ID: 1905)
Streptomyces exfoliatus (Taxon ID: 1905)
Expression System(s):
Method Details:
Experimental Method:
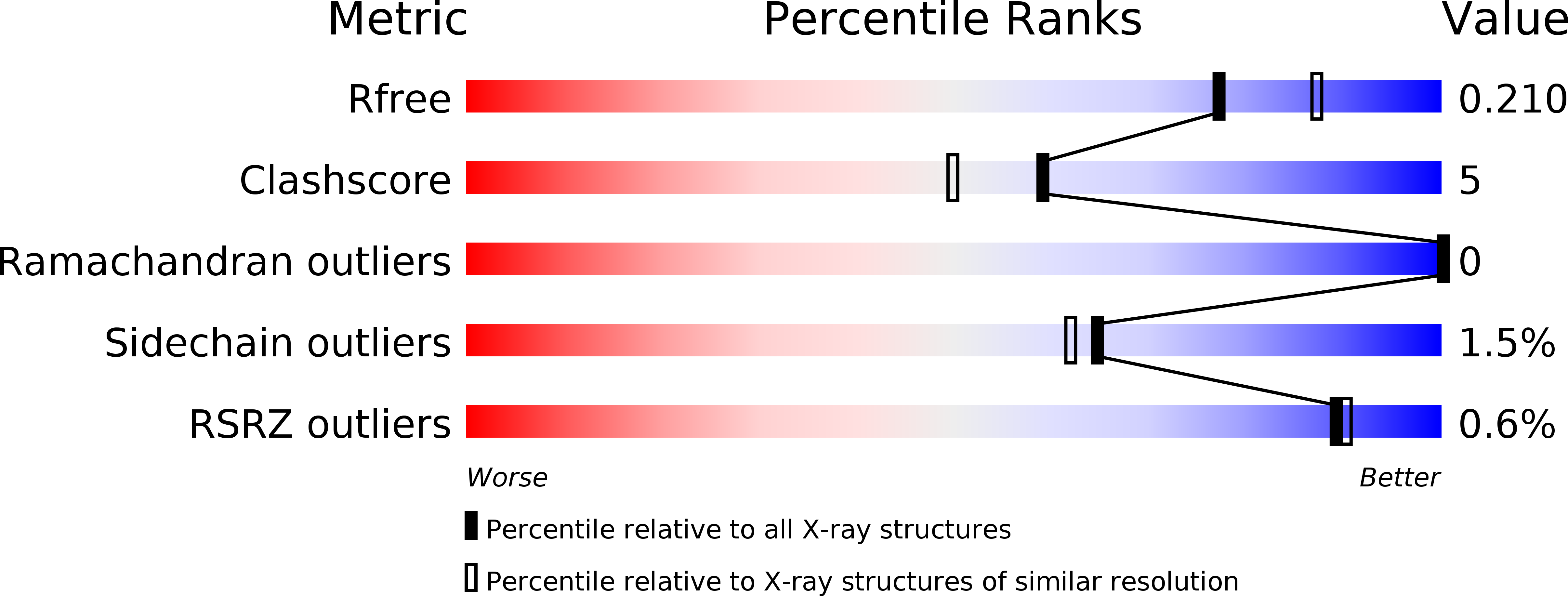
Resolution:
2.06 Å
R-Value Free:
0.21
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
C 1 2 1