Deposition Date
2011-01-07
Release Date
2011-06-08
Last Version Date
2023-11-15
Entry Detail
PDB ID:
3Q9J
Keywords:
Title:
AIIFL segment derived from Alzheimer's Amyloid-Beta displayed on 42-membered macrocycle scaffold
Method Details:
Experimental Method:
Resolution:
2.55 Å
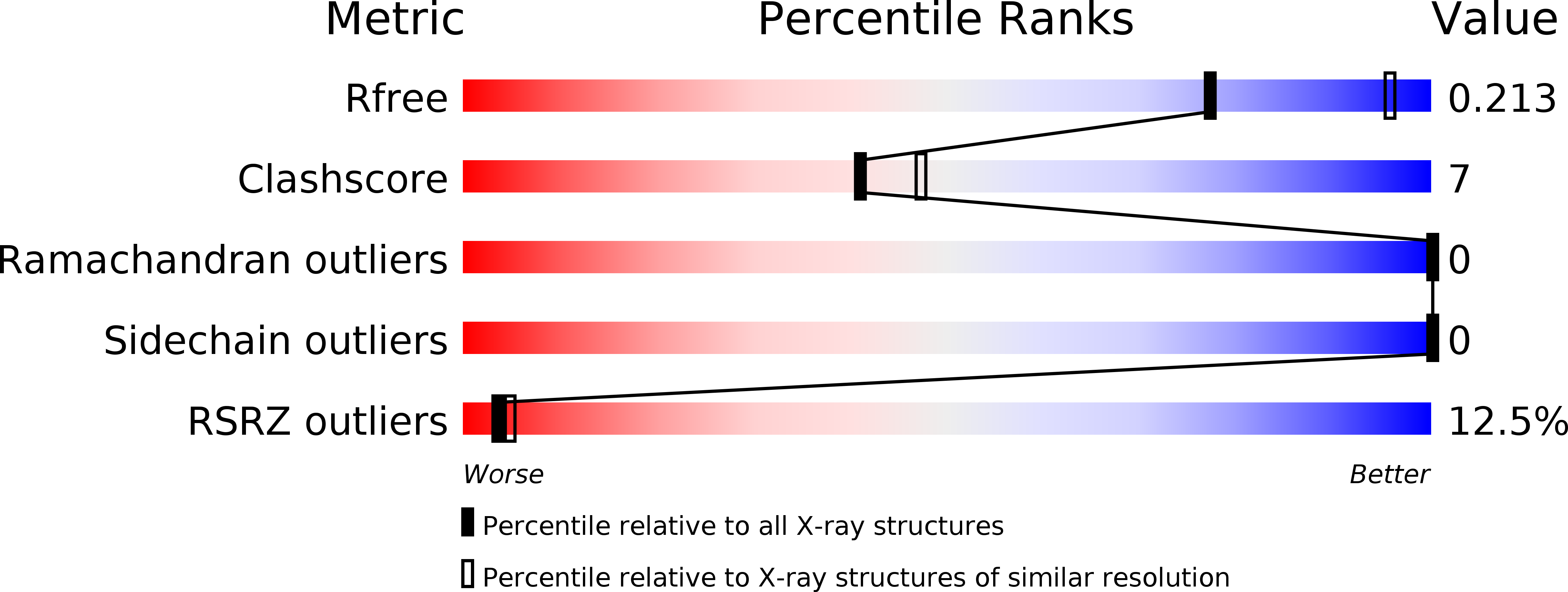
R-Value Free:
0.22
R-Value Work:
0.17
R-Value Observed:
0.18
Space Group:
P 31