Deposition Date
2011-01-05
Release Date
2012-01-11
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3Q7W
Keywords:
Title:
Crystal structure of Symfoil-4P/PV1: de novo designed beta-trefoil architecture with symmetric primary structure, primitive version 1
Method Details:
Experimental Method:
Resolution:
1.50 Å
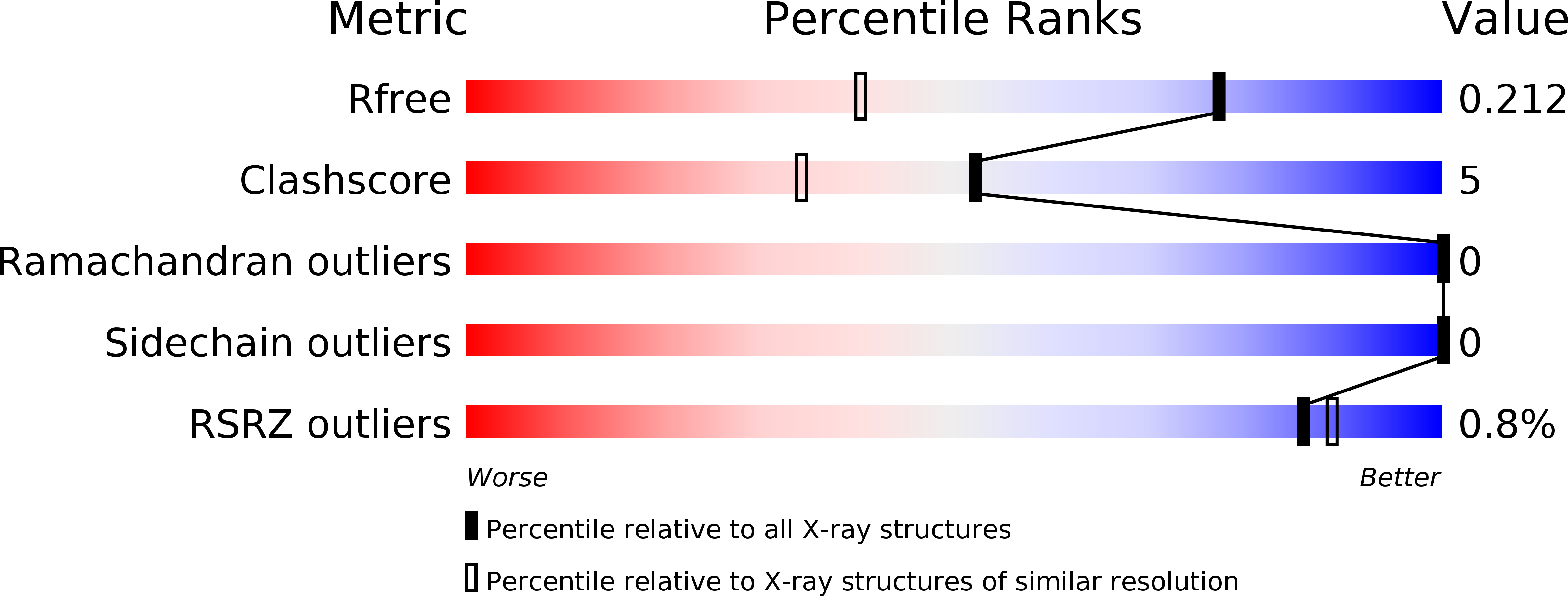
R-Value Free:
0.21
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 21 21 21