Deposition Date
2011-01-01
Release Date
2011-02-16
Last Version Date
2023-09-13
Entry Detail
PDB ID:
3Q6J
Keywords:
Title:
Structural basis for carbon dioxide binding by 2-ketopropyl coenzyme M Oxidoreductase/Carboxylase
Biological Source:
Source Organism(s):
Xanthobacter autotrophicus (Taxon ID: 78245)
Method Details:
Experimental Method:
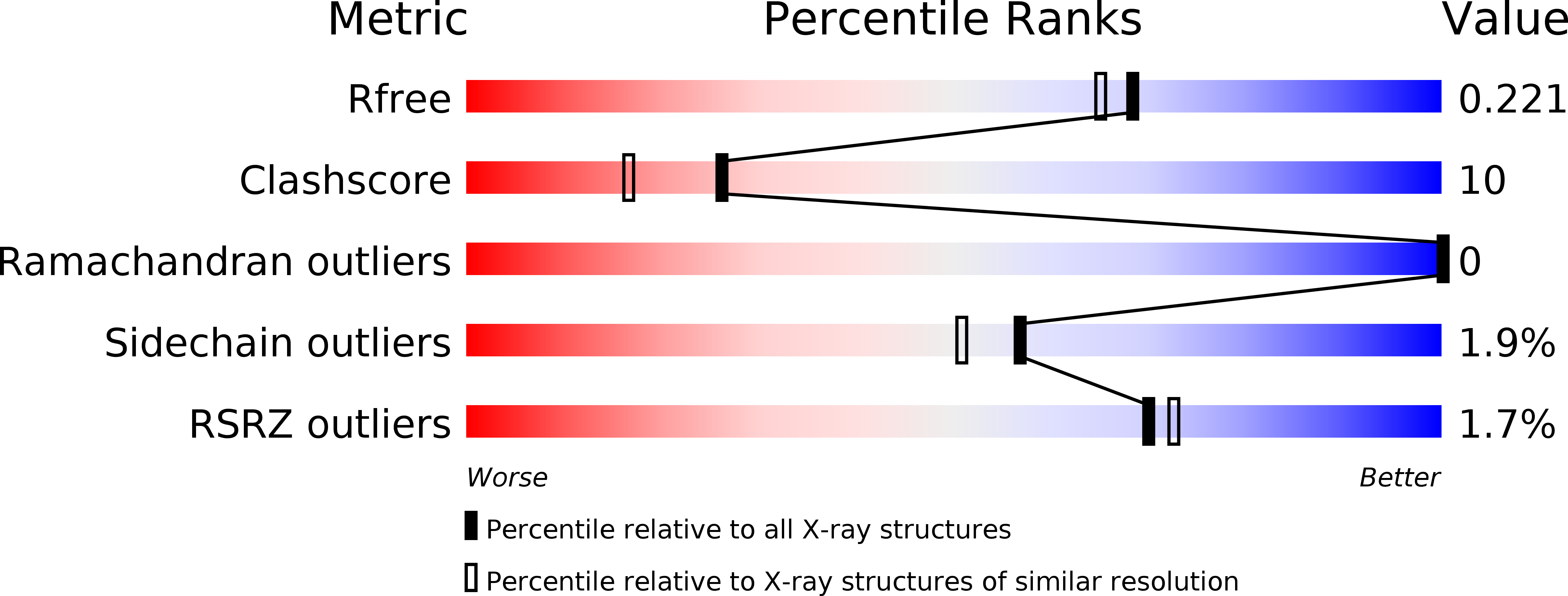
Resolution:
1.92 Å
R-Value Free:
0.22
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 1 21 1