Deposition Date
2010-12-29
Release Date
2011-03-16
Last Version Date
2024-11-20
Entry Detail
PDB ID:
3Q5T
Keywords:
Title:
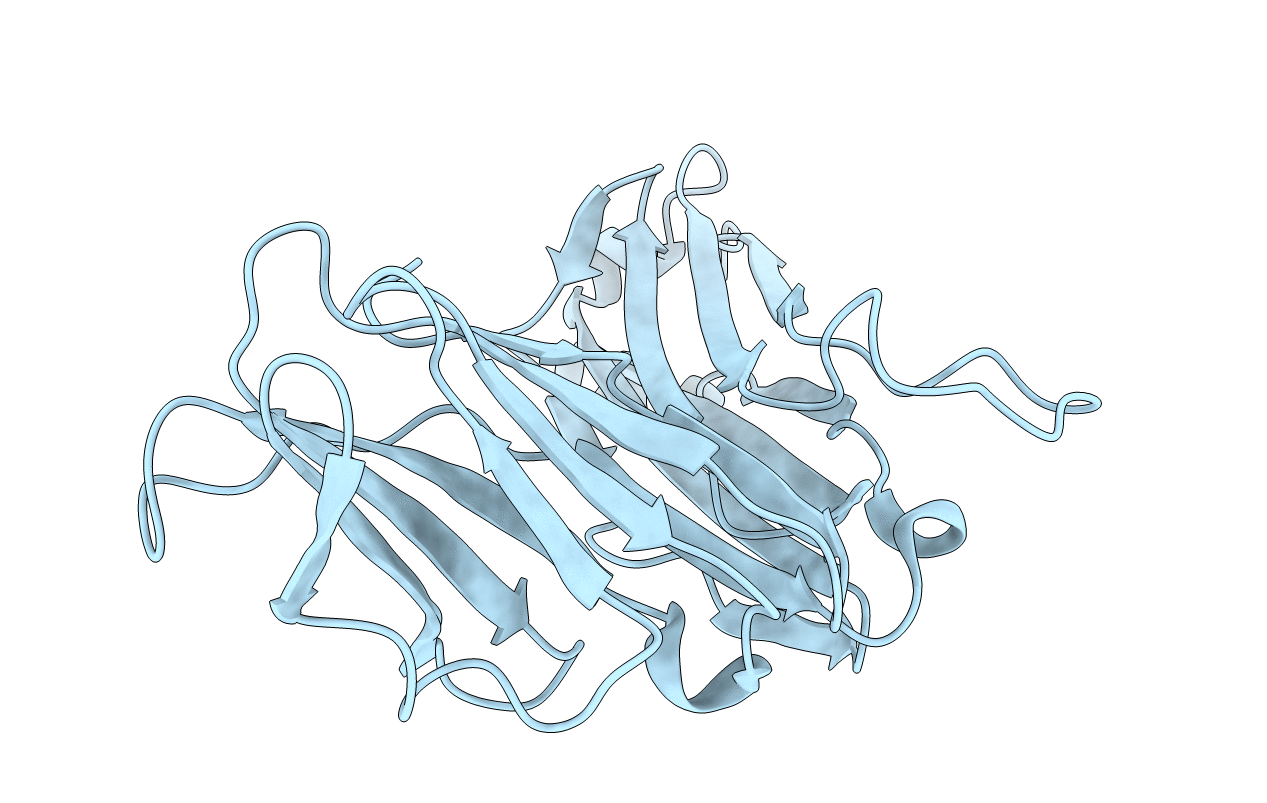
V beta/V beta homodimerization-based pre-TCR model suggested by TCR beta crystal structures
Biological Source:
Source Organism:
Mus musculus (Taxon ID: 10090)
Host Organism:
Method Details:
Experimental Method:
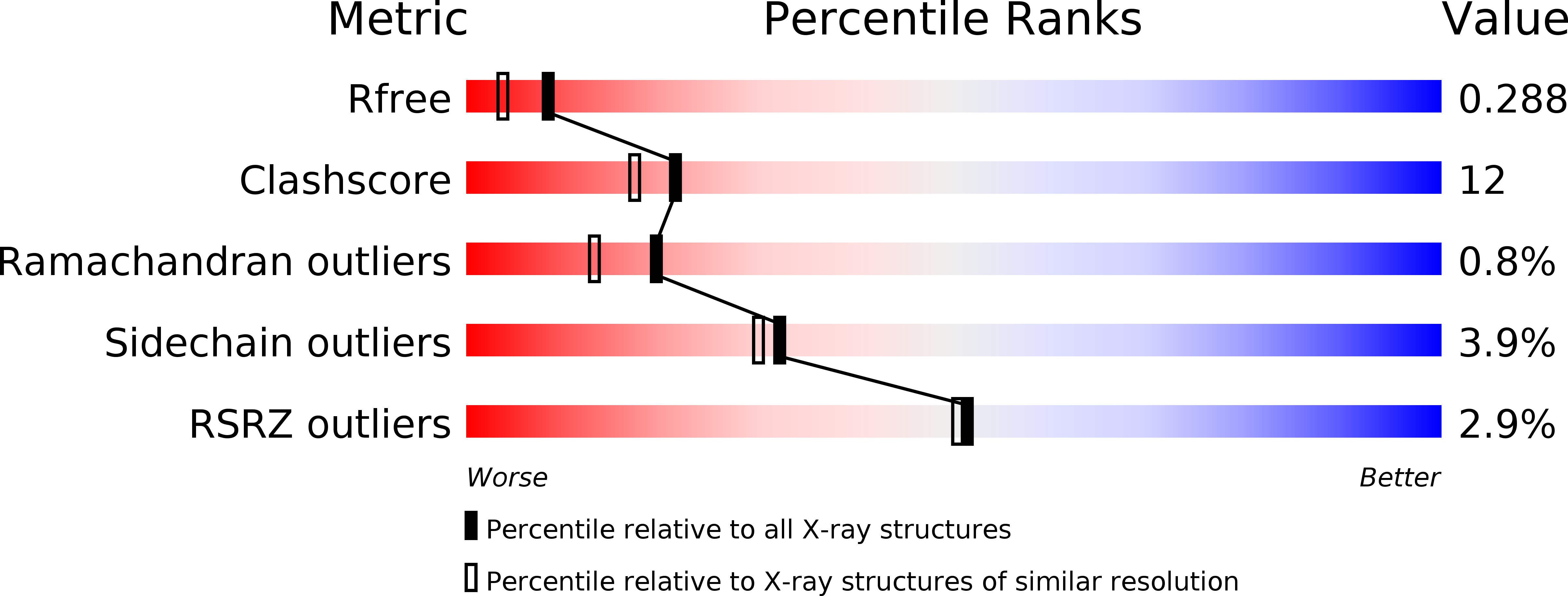
Resolution:
2.01 Å
R-Value Free:
0.28
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
C 2 2 21