Deposition Date
2010-12-13
Release Date
2011-04-06
Last Version Date
2024-02-21
Entry Detail
PDB ID:
3PYJ
Keywords:
Title:
Reduced sweetness of a monellin (MNEI) mutant results from increased protein flexibility and disruption of a distant poly-(L-proline) II helix
Biological Source:
Source Organism(s):
Dioscoreophyllum cumminsii (Taxon ID: 3457)
Expression System(s):
Method Details:
Experimental Method:
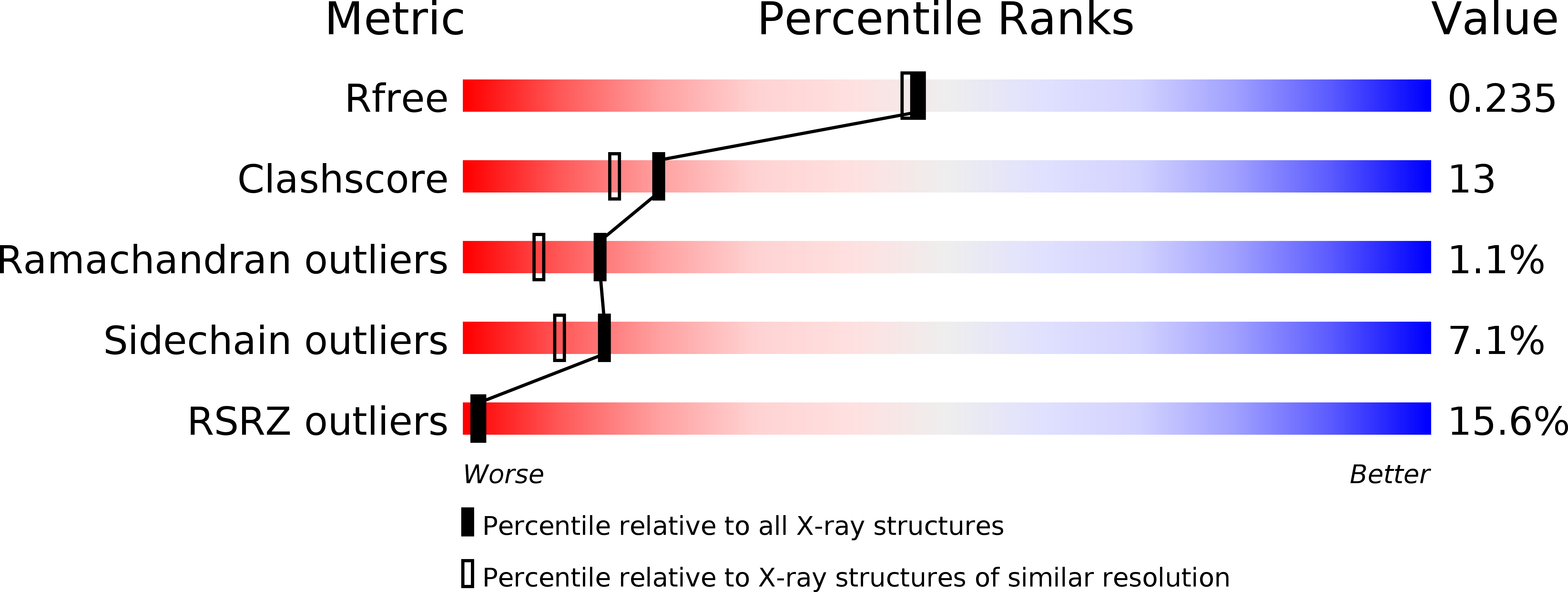
Resolution:
2.00 Å
R-Value Free:
0.27
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 41 21 2