Deposition Date
2010-11-18
Release Date
2011-01-05
Last Version Date
2023-09-06
Entry Detail
PDB ID:
3PN7
Keywords:
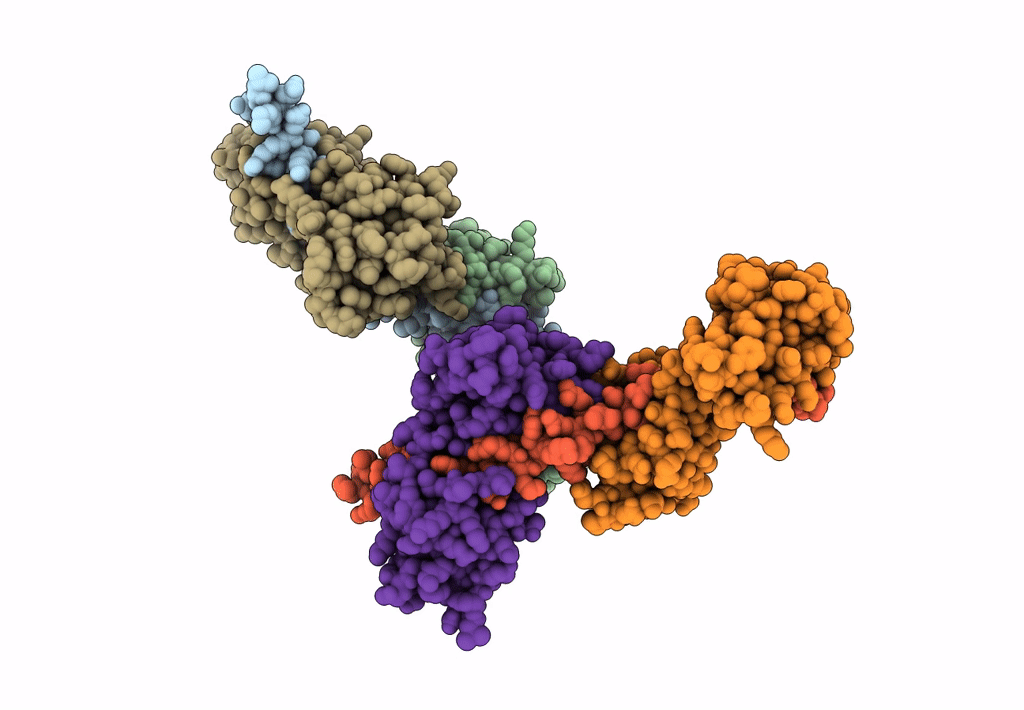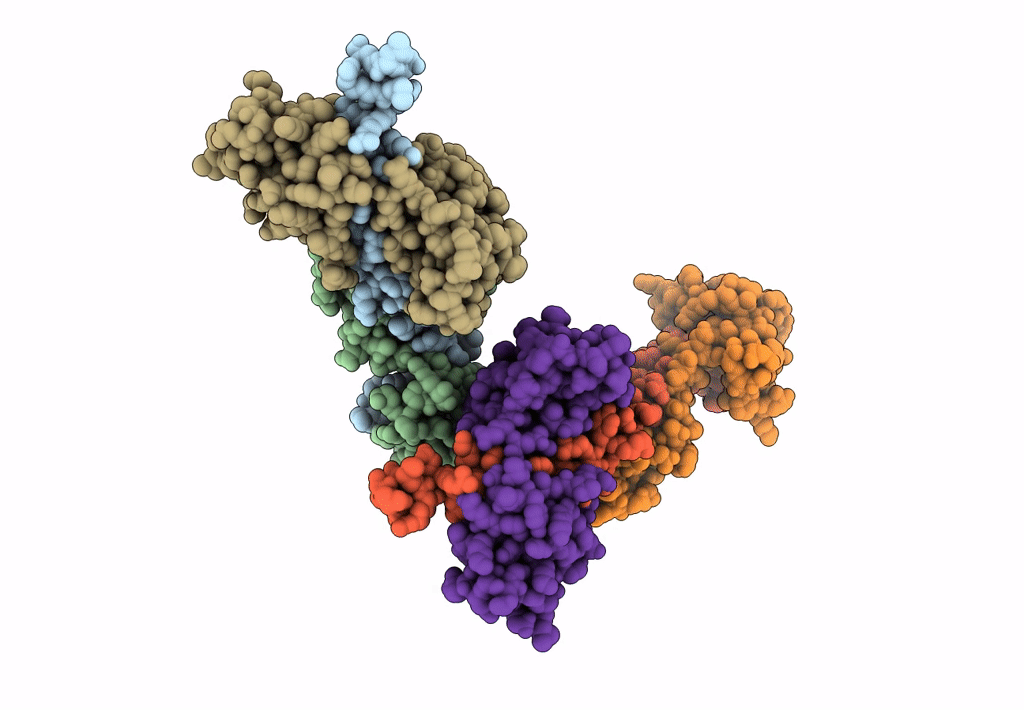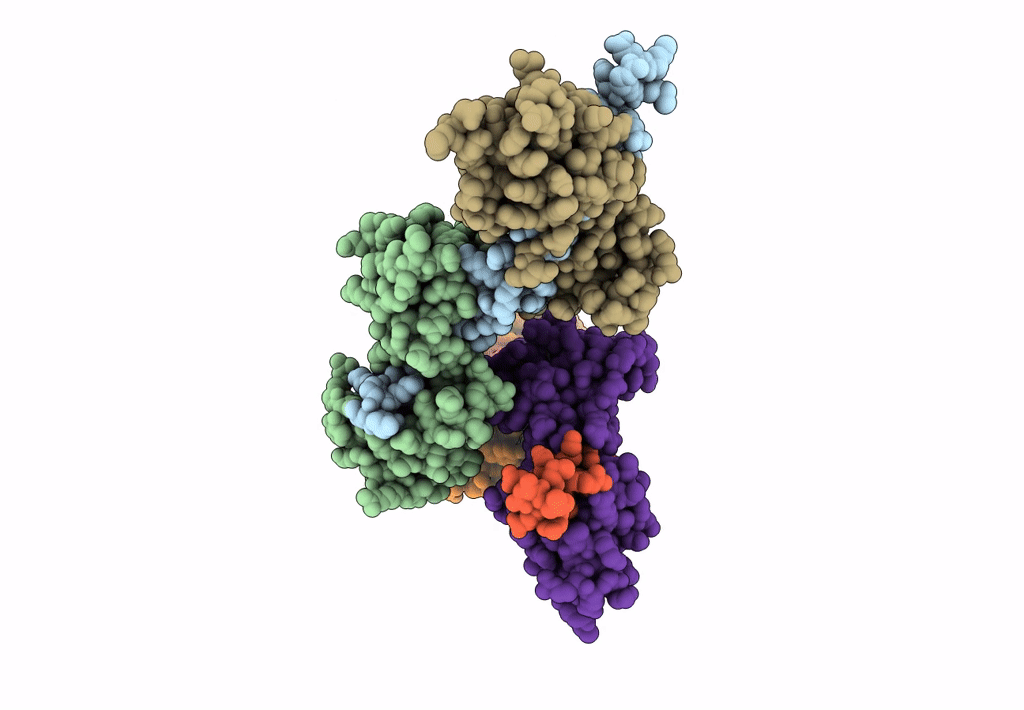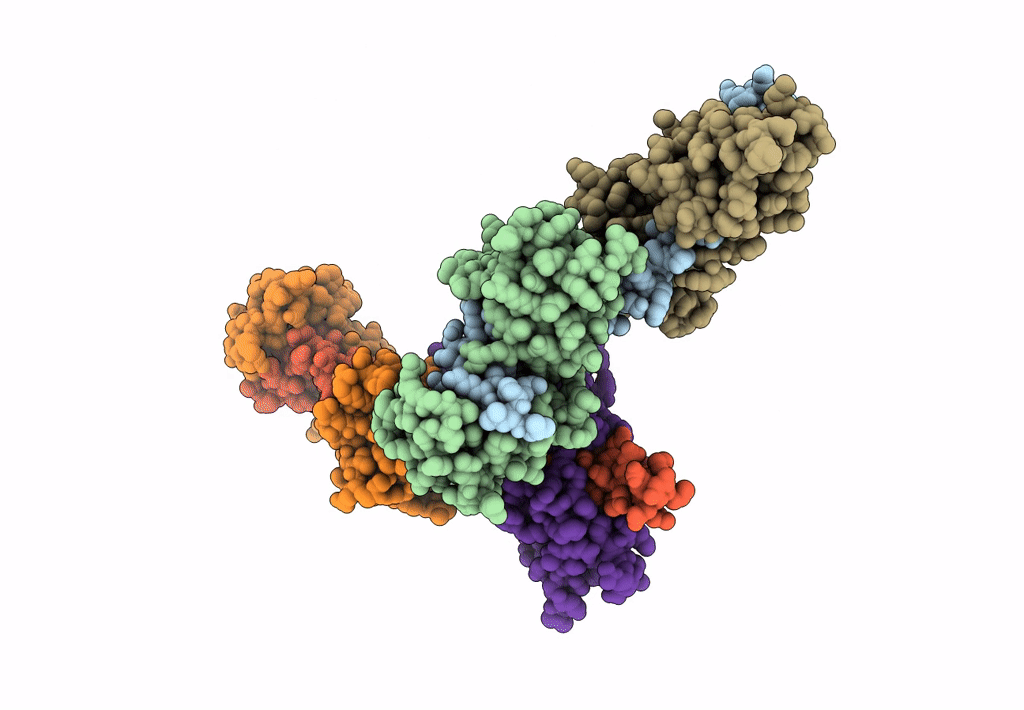
Title:
Visualizing new hinges and a potential major source of compliance in the lever arm of myosin
Biological Source:
Source Organism(s):
Placopecten magellanicus (Taxon ID: 6577)
Method Details:
Experimental Method:
Resolution:
2.25 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1


