Deposition Date
1999-03-22
Release Date
1999-09-01
Last Version Date
2023-09-06
Entry Detail
PDB ID:
3PGT
Keywords:
Title:
CRYSTAL STRUCTURE OF HGSTP1-1[I104] COMPLEXED WITH THE GSH CONJUGATE OF (+)-ANTI-BPDE
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
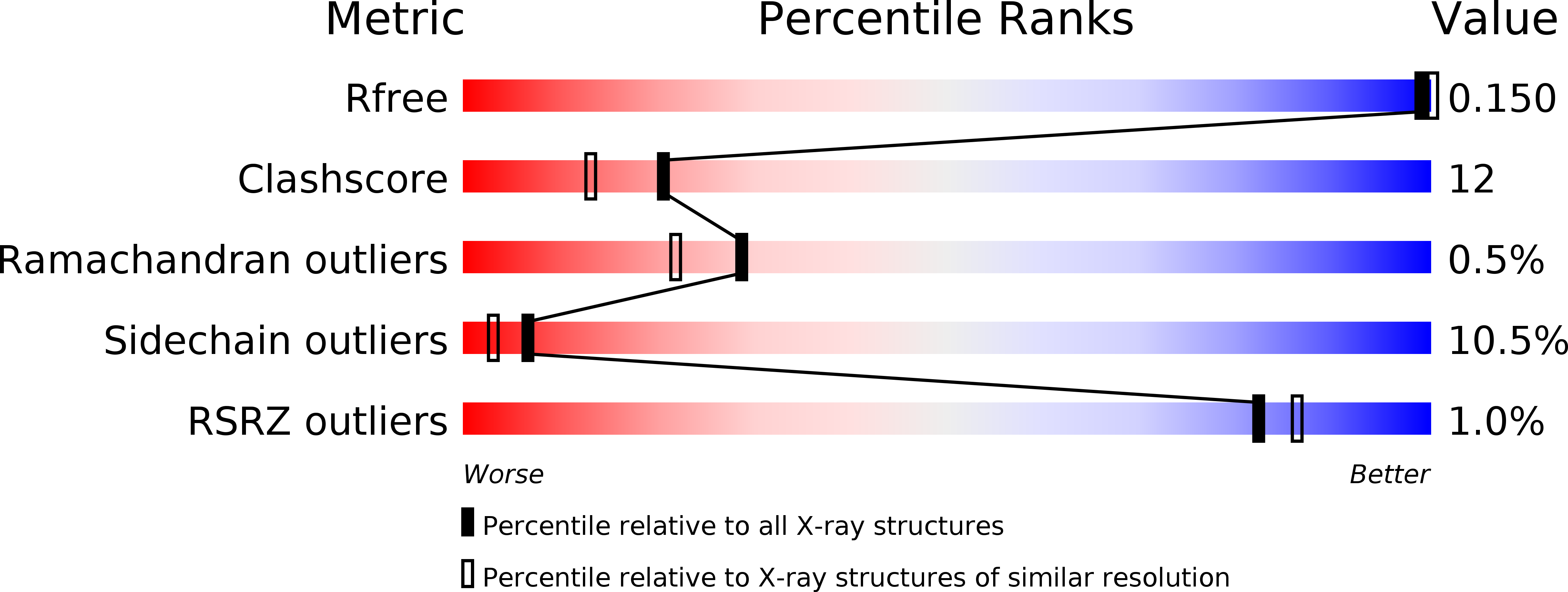
Resolution:
2.14 Å
R-Value Free:
0.15
R-Value Observed:
0.15
Space Group:
C 1 2 1