Deposition Date
2010-08-18
Release Date
2010-09-01
Last Version Date
2024-11-06
Entry Detail
PDB ID:
3OHX
Keywords:
Title:
Molecular Basis for Complement Recognition and Inhibition Determined by Crystallographic Studies of the Staphylococcal Complement Inhibitor (SCIN) Bound to C3c and C3b
Biological Source:
Source Organism:
Staphylococcus aureus (Taxon ID: 158878)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
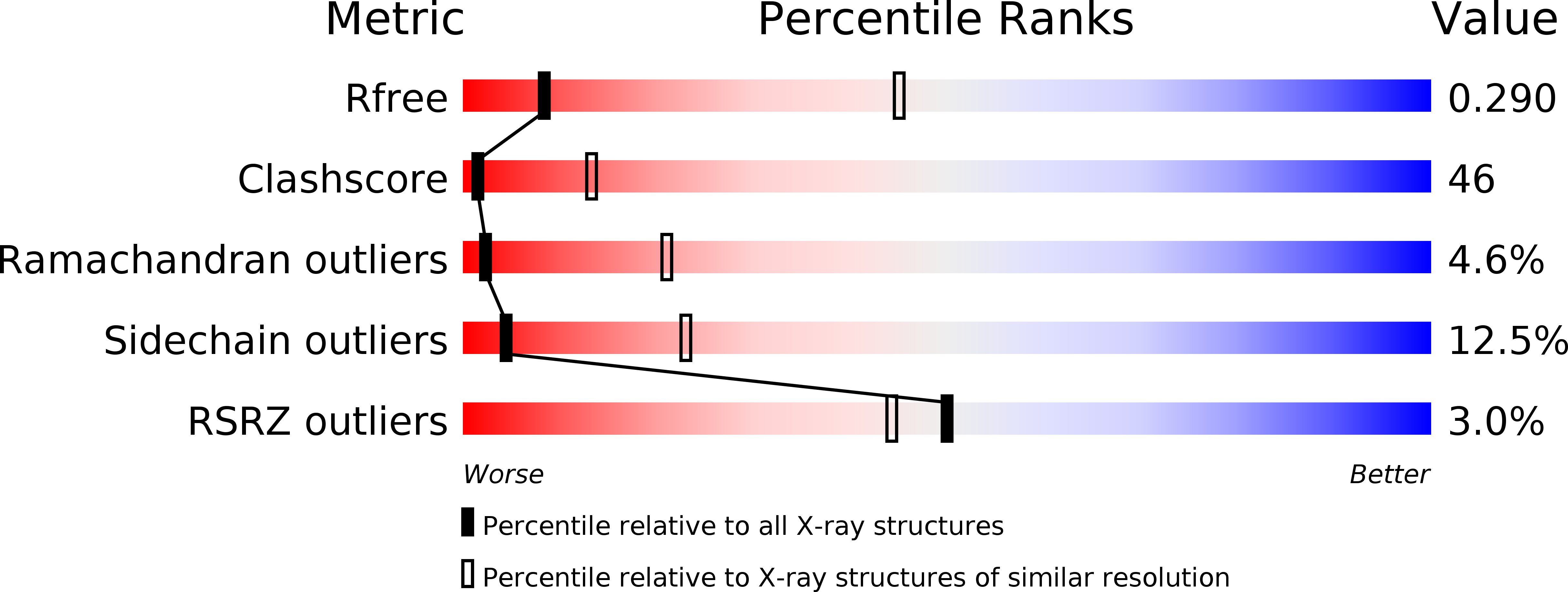
Resolution:
3.50 Å
R-Value Free:
0.29
R-Value Work:
0.27
R-Value Observed:
0.27
Space Group:
P 21 21 2