Deposition Date
2010-07-15
Release Date
2010-07-28
Last Version Date
2023-09-06
Entry Detail
PDB ID:
3NYS
Keywords:
Title:
X-ray structure of the K185A mutant of WbpE (WlbE) from pseudomonas aeruginosa in complex with PLP at 1.45 angstrom resolution
Biological Source:
Source Organism(s):
Pseudomonas aeruginosa (Taxon ID: 208964)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.45 Å
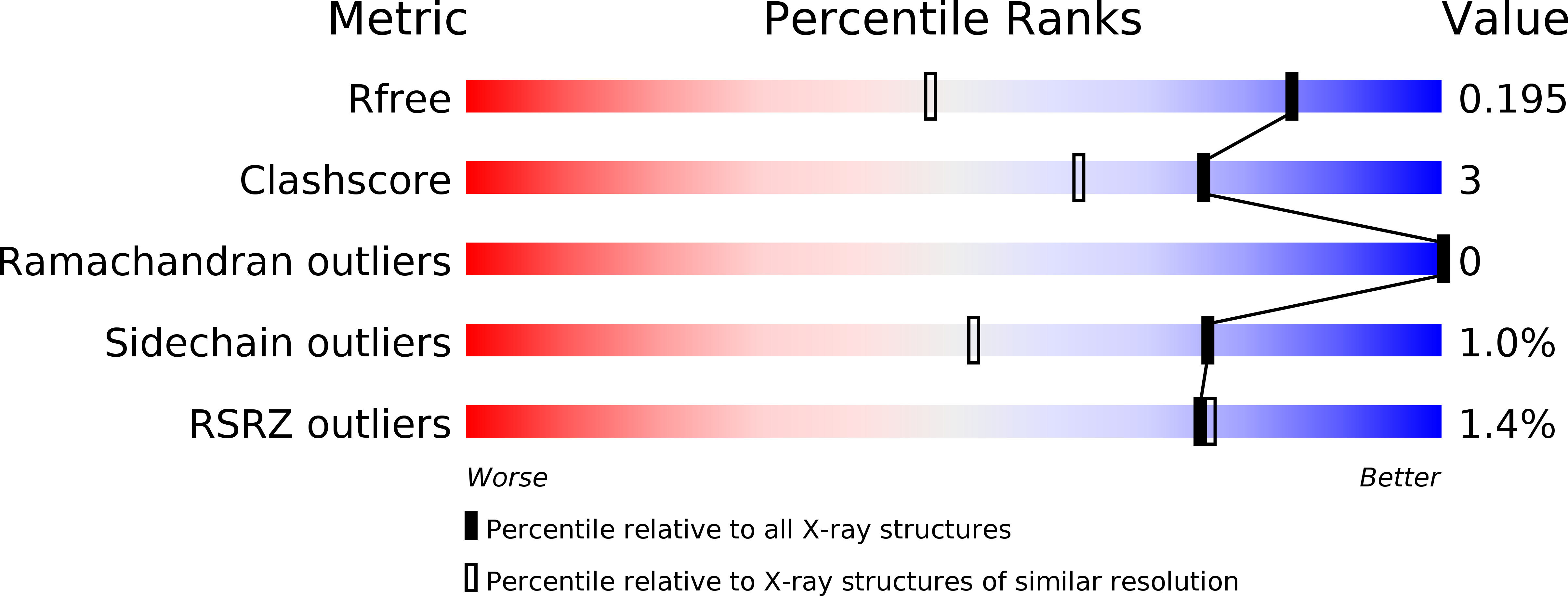
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1