Deposition Date
2010-07-06
Release Date
2010-10-13
Last Version Date
2024-03-20
Entry Detail
PDB ID:
3NU1
Keywords:
Title:
Structure of holo form of a periplasmic heme binding protein
Biological Source:
Source Organism:
Yersinia pestis (Taxon ID: 360102)
Host Organism:
Method Details:
Experimental Method:
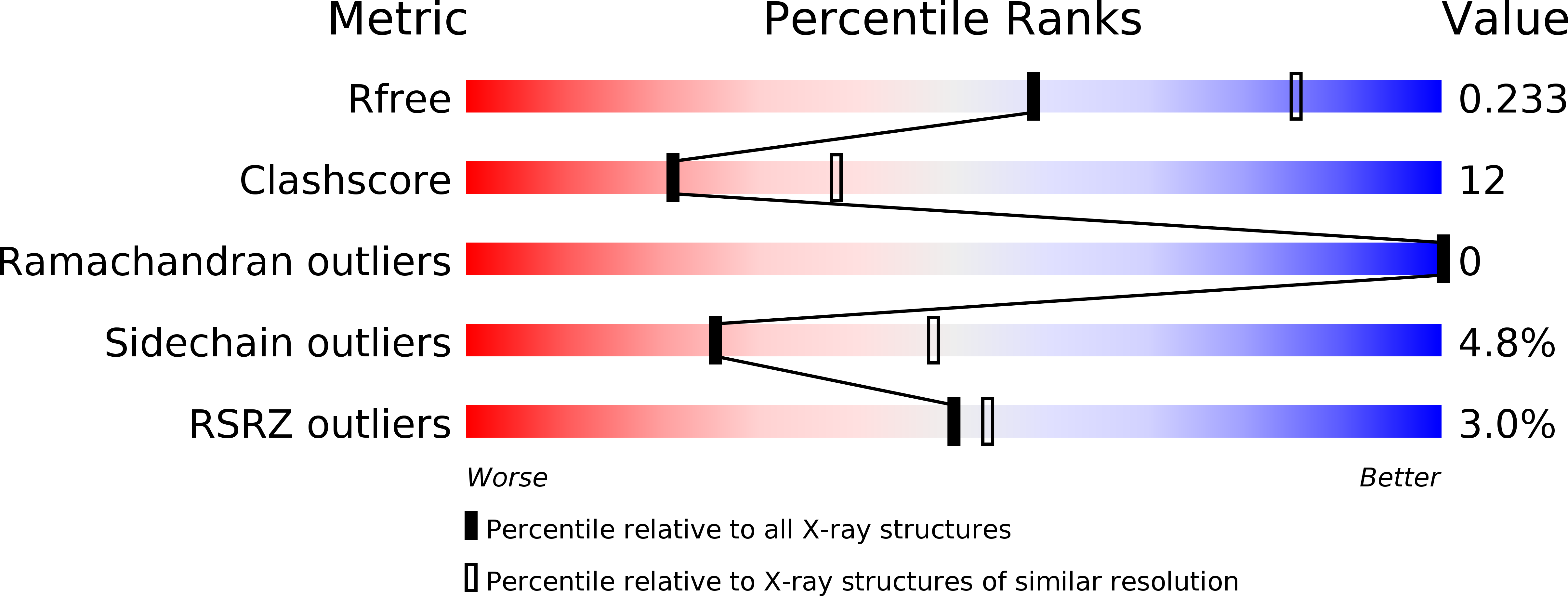
Resolution:
2.50 Å
R-Value Free:
0.23
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 61 2 2