Deposition Date
2010-06-26
Release Date
2010-11-17
Last Version Date
2023-12-27
Entry Detail
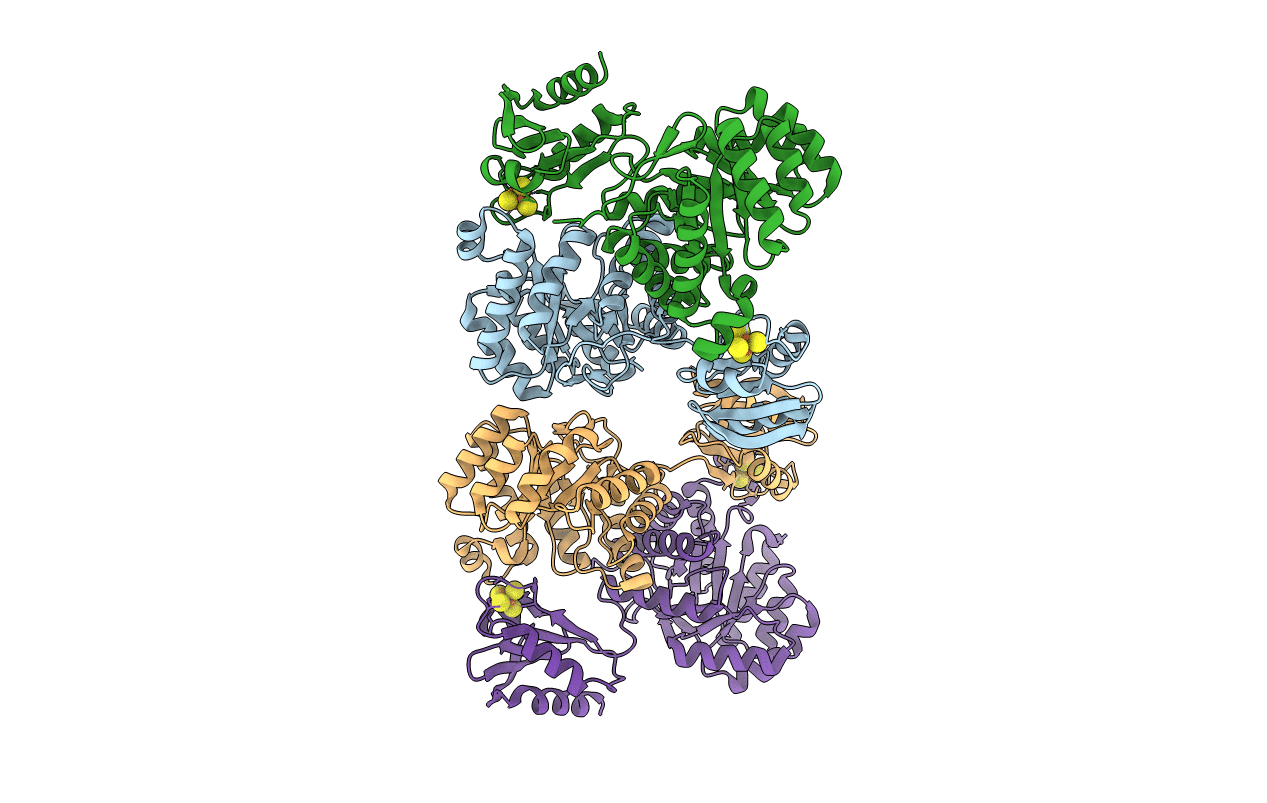
Biological Source:
Source Organism(s):
Aquifex aeolicus (Taxon ID: 63363)
Expression System(s):
Method Details:
Experimental Method:
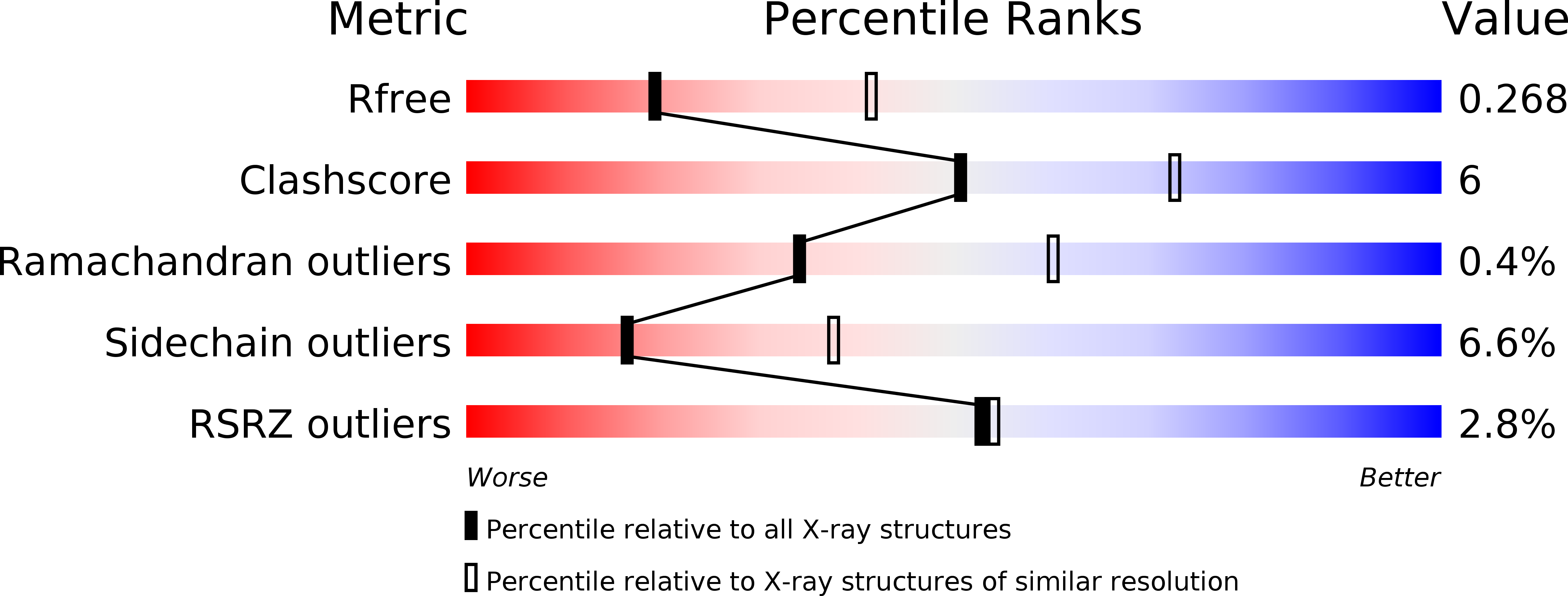
Resolution:
2.70 Å
R-Value Free:
0.26
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
C 1 2 1