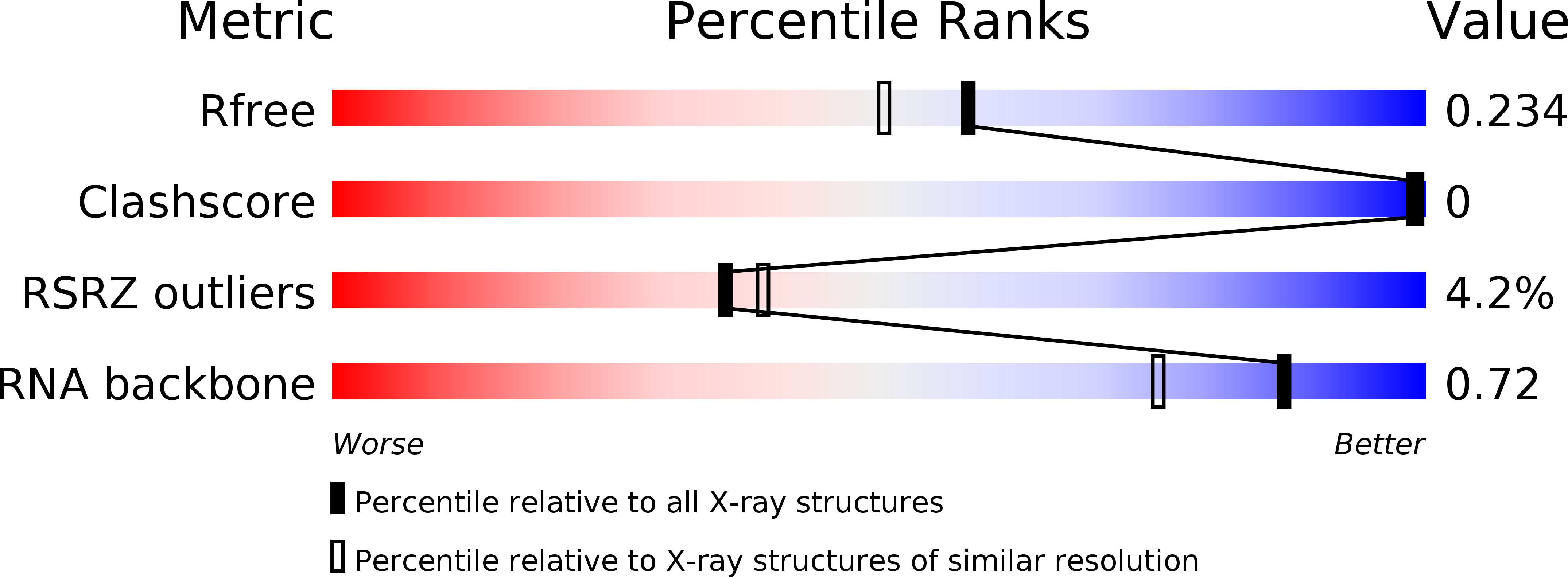
Deposition Date
2010-06-18
Release Date
2010-09-01
Last Version Date
2023-09-06
Entry Detail
PDB ID:
3NKB
Keywords:
Title:
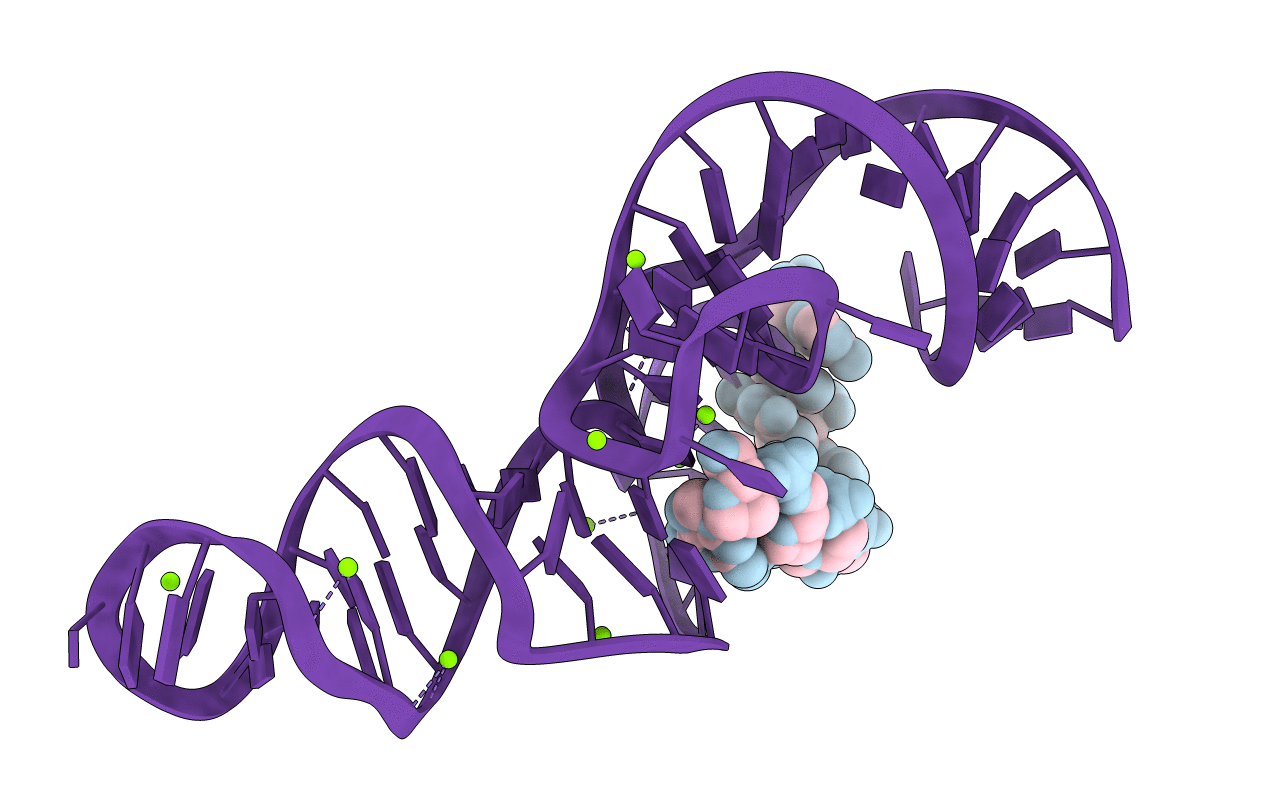
A 1.9A crystal structure of the HDV ribozyme precleavage suggests both Lewis acid and general acid mechanisms contribute to phosphodiester cleavage
Biological Source:
Source Organism(s):
Homo Sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
Resolution:
1.92 Å
R-Value Free:
0.24
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
C 2 2 21