Deposition Date
2010-03-25
Release Date
2010-04-07
Last Version Date
2023-09-06
Entry Detail
PDB ID:
3MBM
Keywords:
Title:
Crystal structure of 2C-methyl-D-erythritol 2,4-cyclodiphosphate synthase from Burkholderia pseudomallei with cytosine and FoL fragment 717, imidazo[2,1-b][1,3]thiazol-6-ylmethanol
Biological Source:
Source Organism(s):
Burkholderia pseudomallei (Taxon ID: 28450)
Expression System(s):
Method Details:
Experimental Method:
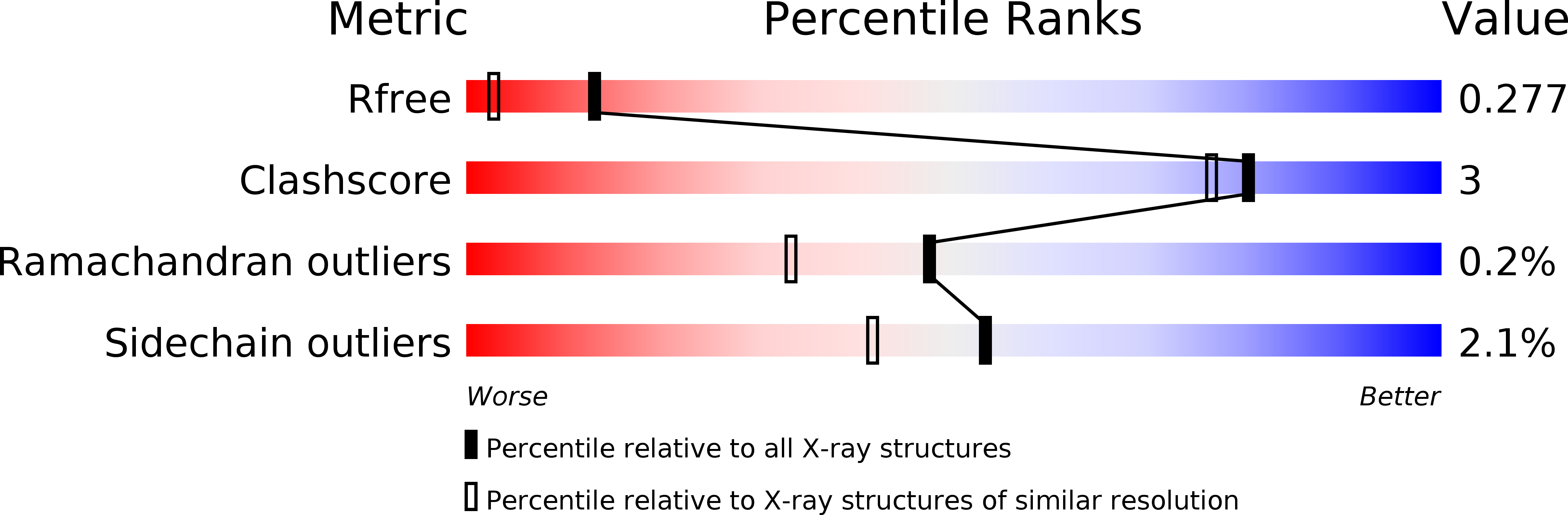
Resolution:
1.80 Å
R-Value Free:
0.21
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
C 1 2 1